How to trace the origin of peritoneal metastasis? A review of treatment strategies
For a long time, it was believed that once tumors metastasized to the peritoneum, they were in the late stage, with limited treatment options and poor prognosis. With the updating of treatment concepts and the establishment of treatment strategies centered on cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC), patients' prognosis and quality of life have been greatly improved, and some patients may even achieve clinical cure.
Release time:
2025-07-08
Source:
* For medical professionals only
For a long time, it has been believed that once a tumor metastasizes to the peritoneum, it is in the late stage, with limited treatment options and poor prognosis. With the updating of treatment concepts and the establishment of a treatment strategy centered on cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC), patients' prognosis and quality of life have been greatly improved, and some patients may even achieve clinical cure.
How to further improve the overall management and prognosis of patients with peritoneal metastasis is a key concern for doctors. Based on recent articles published in journals such as Nat Rev Clin Oncol and Nat Rev Dis Primers, we introduce the progress and future outlook of peritoneal metastasis treatment.
Advances in tumor biology research
Traditional histological classification methods cannot fully express tumor molecular heterogeneity, nor can they guide clinical practice, and they are also inconsistent in prognosis judgment and prediction of treatment response. A deeper understanding of the molecular biology of peritoneal metastasis will help to better understand tumor characteristics, find new biomarkers, and thus improve tumor classification and guide treatment. Molecular markers of common peritoneal metastatic tumors such as gastric cancer, colorectal cancer, and ovarian cancer are shown in Table 1.
Table 1. Molecular markers of peritoneal metastasis

Molecular assessment of chemosensitivity
With the deepening understanding of disease biology, there is also increasing interest in the assessment of chemosensitivity before personalized treatment of peritoneal metastasis. However, this test remains a static assessment and cannot fully predict clinical response. Organoids are a preclinical model that cultivates patient cancer cells in a three-dimensional matrix gel in vitro to simulate the tumor and its microenvironment, thereby achieving personalized chemosensitivity assessment. Organoids have been used to compare different hyperthermic intraperitoneal chemotherapy (HIPEC) regimens in patients with peritoneal metastasis of colorectal cancer and appendiceal tumors, showing significant differences in in vitro cytotoxicity; however, clinical validation has not yet been performed. Immunocheckpoint inhibitor sensitivity can also be assessed using organoid models. It has been reported that designing patient-specific organoids and providing a complete chemosensitivity assessment can be completed in <12 days.
The role of mucus in peritoneal metastasis
Mucinous lesions have a tendency for peritoneal dissemination and can induce chemoresistance by producing extracellular mucin or intracellular components (such as signet ring cells). Changes in mucin expression affect both tumor biology and prognosis. Peritoneal metastasis produces a large amount of mucin, inducing mucinous ascites. Mucin is generally non-degradable, gradually accumulates and prevents immune recognition, thus leading to chemoresistance.
Resectability
When considering curing the disease, the resectability of peritoneal metastasis is a key issue, because the completeness of CRS is the most important prognostic factor. CRS removes all visible tumors in the abdominal cavity through peritoneal resection combined with multi-organ resection. Careful exploration of the abdominal cavity is performed during surgery, and the severity of the disease is assessed using the peritoneal cancer index (PCI). The value of PCI ranges from 0 (no macroscopic tumor lesions) to 39 (large or combined tumor nodules or organ adhesions in all 13 regions in the abdominal cavity), which is currently the most accurate method to describe the distribution of peritoneal tumor metastasis. Postoperative assessment of the extent of cytoreduction must also be determined according to the CC score (CC-0, no residual nodules; CC-1, <2.5 mm; CC-2, <25 mm; and CC-3, >25 mm).
For both primary and recurrent ovarian cancer, the extent of tumor cytoreduction is closely related to patient prognosis. A meta-analysis showed that for every 10% increase in the maximum tumor reduction, the median overall survival (OS) of patients was extended by 5%. Therefore, for ovarian cancer patients, the optimal standard for optimal cytoreduction surgery has changed from residual tumor lesions <1 cm to complete CRS as a first-line or intermittent surgical goal. A randomized trial involving patients with platinum-sensitive recurrent ovarian cancer showed that compared with patients treated with platinum-based drugs alone, complete CRS before chemotherapy helped prolong patient OS (61.9 months vs. 46 months), but incomplete CRS did not benefit (OS, 27.7 months). Similar results have been observed in colorectal cancer and gastric cancer, that is, the more complete the tumor cytoreduction, the better the prognosis.
Improving resectability
Considering that whether CRS is complete is an important prognostic factor for peritoneal metastasis, we should strive to improve its resectability. There is currently a lack of a clear definition of resectability of peritoneal metastasis in the literature, but the most ideal result is to seek a balance between the possibility of cure and long-term survival, acceptable postoperative infection and morbidity risks, and quality of life. Concentrated surgical training for peritoneal metastasis and the establishment of expert centers specializing in the diagnosis and treatment of peritoneal metastasis can improve the complete resection rate of surgery and reduce the risk of perioperative complications and failure-to-rescue. Establishing a multidisciplinary perioperative management methodology and building high-volume centers (more than 45 surgeries per year) can significantly reduce the failure-to-rescue rate.
Resectability assessment
Preoperative tumor resectability assessment is a complex task that requires the cooperation of a multidisciplinary diagnostic and therapeutic team, integrating key information obtained from imaging assessment and laparoscopic staging, and determining the level of resectability in combination with PCI (Figure 1).
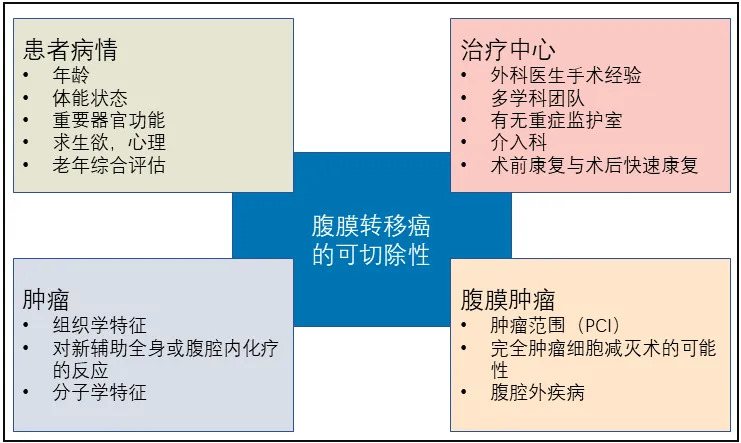
Figure 1. Resectability assessment of radical surgery for peritoneal tumors
CT is the preferred diagnostic method for imaging assessment, which can not only examine peritoneal lesions but also examine the chest cavity to determine whether the tumor has extraperitoneal metastasis (such as lungs or lymph nodes). Peritoneal diffusion-weighted (DW) MRI can better detect lesions located in the small intestine and mesentery, as well as thickening of the peritoneal wall layer and liver capsule. F-FDG contrast-enhanced PET/CT is suitable for initial diagnostic assessment, mainly used to judge the presence or absence of extraperitoneal lesions, especially distant lymph node metastasis, but PET/CT is not suitable for low-metabolic lesions, such as primary mucinous tumors, which may cause false negatives, while for some high-metabolic lesions, PET/CT is prone to false positives.
Preoperative imaging reports should be issued by experienced radiologists, and the reports should include a description of the quantitative method of radiological PCI, and indicate which lesions are unresectable (e.g., those located in the small intestine, mesentery, hepatic hilum, and diffuse pelvic lesions involving the bladder trigone), which lesions may be difficult to resect (due to their anatomical location, it is difficult to achieve ideal cytoreduction), and which lesions are difficult to detect during surgery (hidden in the spleen, liver parenchyma, extraperitoneal space, or abdominal wall).
While imaging has made progress in preoperative resectability assessment, surgical exploration remains the best method for determining the feasibility of CRS. Laparoscopic exploration has become a new option for patients with peritoneal metastasis. Although laparoscopic exploration has many advantages, it may still miss some peritoneal metastases, leading to an underestimation of PCI, especially in areas that have undergone previous surgery, which may be difficult to examine due to adhesions. Laparoscopic exploration causes minimal damage, and occult peritoneal metastases can be found in approximately 20% to 40% of gastric cancer patients. In addition, a comprehensive assessment of the peritoneal cavity can determine whether CRS can be performed laparoscopically.
Perioperative Management
Poor preoperative functional status may increase mortality and morbidity. Perioperative management can improve postoperative outcomes in patients with peritoneal metastasis, reducing the failure rate of rescue from major postoperative complications from 9% to 1%. Three to four weeks before surgery, specialized nutritional, physiological, and psychological preparation should be carried out, mainly from the following four aspects. First, approximately 45% of patients with peritoneal metastasis have malnutrition, which should be corrected. Second, preoperative smoking cessation has been shown to reduce the risk of postoperative morbidity; the pooled risk ratio in meta-analysis was 0.59 (95% CI, 0.41–0.85; P=0.01). Third, physiological preparation can improve the body's functional status and promote recovery. Regular, moderate-intensity exercise can improve inspiratory muscle endurance, reduce postoperative pain scores and anxiety levels, and thus improve overall quality of life. Preoperative chest physiotherapy appears to reduce the incidence of pulmonary complications. Finally, psychological support should be provided to patients, which is crucial for improving their quality of life and compliance.
Most peritoneal metastases occur in people over 70 years old. The outcomes of elderly and younger patients are comparable after complete CRS, but there are significant differences in the incidence and severity of postoperative complications between the two groups. The incidence of serious complications is significantly higher in the elderly population (over 70 years old), and the proportions of postoperative death and rescue failure are also higher. Elderly patients benefit most from rehabilitation support because this population has greater potential for improving physical fitness.
The concept of Enhanced Recovery After Surgery (ERAS) was proposed to promote postoperative recovery. Current ERAS Society guidelines provide a clear pathway for managing patients undergoing CRS. Preoperative rehabilitation plans and ERAS goals are to limit morbidity, complications, and other potential surgical adverse events and shorten the time to initiate scheduled treatment, thereby improving long-term efficacy. A dedicated team should be established to support and coordinate the preoperative rehabilitation plan, including the participation of nurses, fitness coaches, nutritionists, and psychologists.
Intraperitoneal Chemotherapy in the Perioperative Period
The presence of the plasma-peritoneal barrier means that intraperitoneal chemotherapy drugs are cleared much slower than systemic chemotherapy. Therefore, drugs can accumulate locally in the peritoneum, resulting in higher intraperitoneal drug concentrations and longer duration of action, thereby enhancing the therapeutic effect. Intraperitoneal chemotherapy methods include hyperthermic intraperitoneal chemotherapy (HIPEC), pressurized intraperitoneal aerosol chemotherapy (PIPAC), neoadjuvant intraperitoneal and systemic combined chemotherapy (NIPS), and early postoperative intraperitoneal chemotherapy (EPIC).
HIPEC is a multi-parameter treatment that allows for different treatment regimens by altering the administration time, temperature, intraperitoneal pressure level, chemotherapy drug dose, or carrier solution. Due to the numerous parameters, it is difficult to compare the differences between different regimens. Studies in patients with advanced ovarian cancer and gastric cancer have shown that HIPEC combined with CRS is more effective than surgery alone. However, not all tumor patients benefit from HIPEC. The PRODIGE 7 study showed that high-dose oxaliplatin HIPEC + CRS did not benefit patients with colorectal peritoneal metastases, and the incidence of ≥ grade 3 postoperative complications was significantly increased.
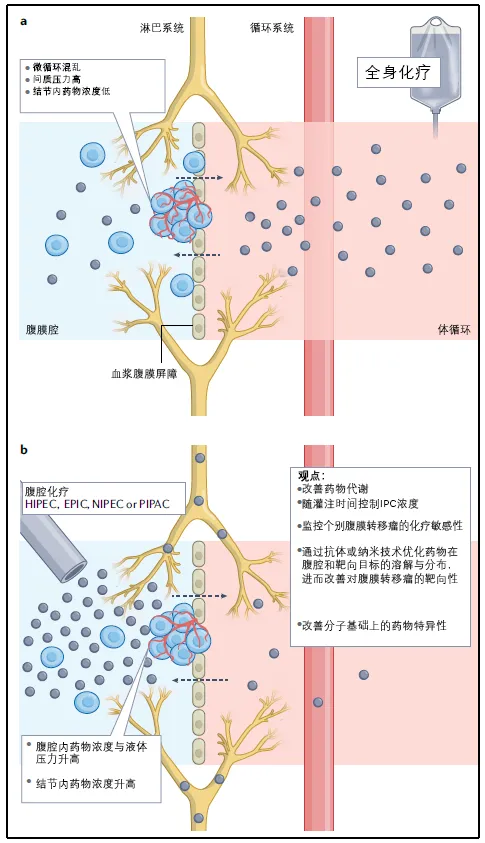
Figure 2. Systemic Chemotherapy vs. Intraperitoneal Chemotherapy
PIPAC is a new therapy that delivers chemotherapy drugs to the peritoneal cavity in an aerosol form through a nebulizer under high pressure generated by a laparoscopic system. The advantages of PIPAC are smaller incisions, higher local drug concentrations, and a lower risk of systemic adverse events. Since the first use of PIPAC in 2011, several cohort studies have demonstrated good safety and tolerability of PIPAC for various PSM patients. PIPAC is mainly used in combination therapy, such as alternating with systemic chemotherapy. PIPAC is not currently considered a standard treatment for any condition, although it is often used for palliative treatment in patients who do not meet the criteria for CRS. PIPAC has been shown to induce histological regression of gastrointestinal or ovarian peritoneal metastasis. In addition to its role in palliative treatment, PIPAC combined with systemic chemotherapy can allow some gastric cancer patients to reach the resection threshold and undergo surgery + HIPEC. As a safe delivery platform, the nebulization method used in PIPAC may be suitable for delivering novel formulations (such as nanoparticle formulations or viral formulations) as well as targeted therapy and immunotherapy drugs.
Systemic Treatment
Peritoneal cancer generally has a poor prognosis, which is related to its resistance to systemic chemotherapy or targeted therapy. The poor efficacy of systemic treatment comes from multiple factors: the peritoneal-plasma barrier makes it difficult for systemically administered drugs to reach peritoneal lesions; insufficient blood supply within the peritoneum leads to tumor hypoxia and low levels of apoptosis and programmed cell death, which may affect the activity of anticancer drugs; increased interstitial pressure within the tumor limits drug entry into tumor cells; intracellular or extracellular mucin may confer a degree of chemoresistance; and the specific immune microenvironment of peritoneal metastasis may affect the activity of anticancer immunotherapy.
Systemic treatment is usually not intended for radical cure: palliative treatment aims to prolong patient survival and improve quality of life by controlling the disease and its symptoms; the purpose of perioperative systemic treatment is to eliminate micrometastases not removed by surgery, thereby increasing the possibility of radical cure.
Systemic chemotherapy is an important component of peritoneal metastasis treatment. The chemotherapy regimen depends on the primary tumor, the extent of peritoneal spread, the choice of CRS, and the patient's condition and organ function. In addition, targeted drugs and immunotherapy have been widely used in the treatment of other metastatic tumors, but there is currently a lack of clinical trials of systemic targeted therapy and immunotherapy for peritoneal metastasis, and knowledge in this area is limited.
Endocrine therapy, including selective estrogen receptor modulators, estrogen receptor blockers, and aromatase inhibitors, combined with cell cycle arrest drugs CDK4/6 inhibitors, can be used to treat hormone-dependent tumors such as breast cancer. HER2-targeted therapy can be used to treat HER2+ breast cancer and gastric cancer, PARP inhibitors can be used to treat tumors carrying HRD, such as epithelial ovarian cancer or pancreatic ductal adenocarcinoma with BRCA mutations. Immune checkpoint inhibitors are used to treat patients with microsatellite instability-high colorectal cancer and non-colorectal cancer.
Targeted therapy and immunotherapy are rapidly developing. Patients should be encouraged to participate in clinical research, and treatment centers should establish multidisciplinary teams to discuss patient molecular stratification and personalized treatment options to ensure optimal treatment outcomes.
Treatment of Specific Peritoneal Metastasis Cancer
The management strategies for peritoneal metastasis vary depending on the primary tumor. Multidisciplinary treatment usually involves perioperative chemotherapy, surgery, and intraperitoneal chemotherapy.
Colorectal Peritoneal Metastasis
For colorectal peritoneal metastasis, CRS + mitomycin C-based HIPEC after systemic chemotherapy significantly prolongs patient survival compared to systemic chemotherapy alone (22.4 months vs. 12.6 months; P = 0.032). In addition, patients who received intraperitoneal chemotherapy after CRS had longer survival than those who received only systemic chemotherapy (25 months vs. 18 months; P = 0.04). Given the results of the PRODIGE7 study, the role of HIPEC in colorectal peritoneal metastasis remains controversial.
Gastric Peritoneal Metastasis
Peritoneal metastasis is common in advanced gastric cancer patients, and their prognosis remains poor despite systemic chemotherapy. Analysis of prospective databases shows that HIPEC + CRS benefits survival in carefully selected patients with focal peritoneal metastasis. For unresectable gastric peritoneal metastasis, palliative intraperitoneal chemotherapy has shown encouraging results. Systemic chemotherapy combined with cisplatin and doxorubicin-based PIPAC resulted in a median OS of 19.1 months, and 14.3% of patients met the criteria for radical surgery. Docetaxel-based NIPS or palliative intraperitoneal chemotherapy appears to further alleviate peritoneal progression and improve survival.
Ovarian and Tubal Peritoneal Metastasis
CRS and perioperative systemic chemotherapy are standard treatments for ovarian and tubal peritoneal metastasis. When patients cannot undergo complete CRS due to severe illness or poor physical condition, 3-4 cycles of neoadjuvant systemic chemotherapy can be considered before reassessing the possibility of complete CRS. HIPEC combined with CRS is promising, especially for platinum-resistant ovarian cancer. A randomized trial found that the median OS after systemic chemotherapy was 19.4 months with and 11.2 months without HIPEC after CRS (P < 0.05).
The efficacy and safety of cisplatin and doxorubicin-based PIPAC for recurrent ovarian and tubal cancer peritoneal metastasis have been verified in phase 1 trials: 62% of patients achieved objective remission; 76% of patients receiving 3 courses of PIPAC showed histological remission and PCI improvement; no treatment-related grade 4 adverse events or deaths were observed.
Rare Peritoneal Metastasis
Rare peritoneal metastasis refers to peritoneal metastasis from tumors that rarely metastasize to the peritoneum or rarely meet the criteria for radical resection (mainly due to extraperitoneal metastasis), including pancreatic cancer, cholangiocarcinoma, breast cancer, lung cancer, neuroendocrine tumors, and sarcomas.
For pancreatic cancer peritoneal metastasis, the OS of two patients treated with CRS + mitomycin C-based HIPEC was 48 months and 70 months, respectively, but the OS of seven patients treated with CRS + cisplatin-based HIPEC was only 16 months. For cholangiocarcinoma peritoneal metastasis, a study analyzed data from 34 patients who received CRS + HIPEC and 21 patients who received systemic chemotherapy, with OS of 21.4 months and 9.3 months, respectively. Breast cancer peritoneal metastasis is extremely rare. One study included 5 patients who received CRS + HIPEC, with a median time of 18 years between breast cancer diagnosis and peritoneal metastasis, and a patient OS of 56 months. Neuroendocrine tumor peritoneal metastasis often has a poor prognosis. If complete resection is possible, these patients should receive CRS at high-volume centers. The role of HIPEC in these patients is unclear.
Prophylactic Treatment of Peritoneal Metastasis
Theoretically, peritoneal metastasis can occur in tumors originating from any tissue, with more research on CRC and gastric cancer peritoneal metastasis. Clinical manifestations (such as digestive tract obstruction, perforation, disease progression to locally advanced stage, positive peritoneal cytology) and pathological features (such as mucinous or signet ring cell carcinoma, severe lymph node invasion) can be combined to assess the risk of peritoneal metastasis.
Surgical resection, enhanced systemic therapy, and local combined therapy have all been proposed as strategies to prevent peritoneal metastasis, but their effectiveness is questionable. Systemic surgical exploration was proposed 50 years ago to detect and remove peritoneal metastasis, but the success rate is limited. Enhanced adjuvant systemic chemotherapy regimens with three or four drugs have been proposed for the prevention of peritoneal metastasis due to their effectiveness in metastatic diseases. The IROCAS study attempts to test the effectiveness of this strategy. This study will compare the effects of adjuvant mFOLFIRINOX and mFOLFOX6 in patients with pT4N1 or pT1-4N2 colon cancer. Patient enrollment is almost complete. However, given the resistance of peritoneal metastasis to FOLFOX-based chemotherapy, the effectiveness of this regimen remains questionable.
The COLOPEC and PROPHYLOCHIP randomized controlled trials investigated the efficacy of local combined therapy. Secondary exploration + short-term HIPEC + high-dose oxaliplatin in patients with locally advanced colorectal cancer did not successfully prevent peritoneal metastasis. In both studies, a considerable proportion of patients (12% and 11%) were found to have peritoneal metastasis during surgical exploration, despite pre-operative examinations excluding peritoneal metastasis. The results of the MILAN study were encouraging, with a 5-year cumulative incidence of peritoneal metastasis of 9.3% in the HIPEC group and 42.5% in the control group. In the subgroup analysis of COLOPEC, patients with right-sided colorectal cancer who received HIPEC therapy showed near-significant benefit, with an 18-month peritoneal metastasis-free survival rate of 81% in the experimental group and 65% in the control group (P = 0.064).
Researchers have explored the effectiveness of various prophylactic HIPEC regimens in patients with advanced gastric cancer, showing that it can reduce the incidence of peritoneal metastasis and improve OS, but controversies surrounding this therapy still exist, and more research results are needed for confirmation.
Future Outlook
Personalized treatment
Patient-derived organoids can be used to detect patient sensitivity to chemotherapeutic drugs and predict treatment response and resistance. Organoid technology holds promise for personalized intraperitoneal chemotherapy, but clonal pressure and tumor cell heterogeneity are obstacles to its large-scale application. In addition, other chemotherapy sensitivity test models have also been developed, including xenograft models, 2D cell monolayers, 3D in vitro tumor models, etc.
Nanomedicines for Intraperitoneal Therapy
The main drawbacks of intraperitoneal therapy are the rapid clearance of chemotherapeutic drugs from the peritoneal cavity and into the systemic circulation, and low specificity for tumor cells. Nanomaterials are widely used as drug molecule carriers, such as small molecule drugs, proteins, or nucleic acids. Nanoparticle albumin-bound paclitaxel and liposomal doxorubicin have been approved for clinical cancer treatment. Sustained-release systems loaded with nanomedicines and delivery of nanoparticle drugs via PIPAC technology have shown promise in animal experiments. The challenges of the former strategy lie in the large peritoneal surface area, making it difficult to achieve uniform distribution of nanoformulations, while also avoiding drug adhesion to tissues causing inflammation. The latter strategy allows for uniform drug distribution in the peritoneal cavity, but it remains unclear whether it is as effective as chemotherapy and whether it can be used for nucleic acid drugs.
Novel Surgical Techniques
Even if CRS achieves CC-0, and the peritoneum appears completely normal to the naked eye, there may be residual malignant lesions, so more precise peritoneal resection is needed. Near-infrared fluorescence-guided surgery has enormous potential in the field of peritoneal metastasis, and this technology can use targeted or "smart" fluorescent dyes to detect cancerous tissue in real time. Indocyanine green can improve the detection rate of peritoneal metastasis in colorectal cancer patients by 30%. Using a fluorescent dye targeting FRA (a molecule overexpressed in 95% of epithelial ovarian cancers), surgeons detected nearly 5 times more tumor nodules than with standard observation.
References
[1] Kepenekian V,Bhatt A,Péron J,et al.Advances in the management of peritoneal malignancies.Nat Rev Clin Oncol 2022;19:698-718.
[2] Cortés-Guiral D,Hübner M,Alyami M,et al.Primary and metastatic peritoneal surface malignancies.Nat Rev Dis Primers 2021;7:91.
Source: NEJM Medical Frontier