Chinese Expert Consensus on Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2025 Edition)
Colorectal cancer is one of the most common malignant tumors in China, ranking second and fourth in incidence and mortality rates among all malignant tumors, respectively. Metastasis and recurrence are the main causes of death in patients with colorectal cancer. The peritoneum is a common site of colorectal cancer metastasis, second only to liver and lung metastasis, but its prognosis is far worse than that of liver and lung metastasis.
Release time:
2025-07-09
Source:
Colorectal Cancer Professional Committee, Chinese Medical Doctor Association
Corresponding author: Xiong Bin, Email: binxiong1961@whu.edu.cn; Wang Xishan, Email: wxshan1208@126.com
Abstract: Colorectal cancer (CRC) is one of the most common malignant tumors in China, ranking second in incidence and fourth in mortality among all malignant tumors. Metastasis and recurrence are the leading causes of death in CRC patients. Peritoneal metastasis is a common site of CRC metastasis, second only to liver and lung metastases, but its prognosis is far worse than that of liver and lung metastases. Early diagnosis of peritoneal metastasis of CRC is difficult, with severe symptoms and poor prognosis. Standardized diagnosis and treatment are crucial for improving patient prognosis and quality of life. China
The Colorectal Cancer Professional Committee of the Chinese Medical Doctor Association organized domestic authoritative experts in the field of colorectal cancer to compile the "Chinese Expert Opinion on the Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2017 Edition)" as early as 2017, and revised it in 2022. In view of the research progress on peritoneal metastasis of colorectal cancer, the Colorectal Cancer Professional Committee of the Chinese Medical Doctor Association organized multidisciplinary experts from across the country to revise the "Chinese Expert Opinion on the Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2017 Edition)" and the 2022 revision based on the latest research results and evidence-based medical evidence. After repeated discussions, the "Chinese Expert Consensus on the Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2025 Edition)" was compiled, reaching a preliminary consensus on the definition, diagnosis, treatment, and prevention of colorectal cancer peritoneal metastasis, in order to guide and standardize the diagnosis and treatment of colorectal cancer peritoneal metastasis, formulate reasonable and effective comprehensive treatment plans, prolong the survival time and improve the quality of life for patients with colorectal cancer peritoneal metastasis, and thus improve the overall level of diagnosis and treatment of colorectal cancer in China.
Keywords: colorectal neoplasms; peritoneal metastasis; diagnosis; treatment; consensus
Chinese expert consensus on the diagnosis and treatment of colorectal cancer peritoneal metastasis (2025 edition)
Colorectal Cancer Professional Committee, Chinese Medical Doctor Association
Corresponding to: Xiong Bin, Email: binxiong1961@whu.edu.cn; Wang Xishan, Email: wxshan1208@126.com
Abstract: Colorectal cancer (CRC) is one of the most common malignant tumors in China, ranking second in morbidity and fourth in mortality. Metastasis and recurrence are the leading causes of CRC patient death, and the peritoneum is a frequent site of metastasis in CRC, second only to liver and lung metastases. However, the prognosis of peritoneal metastasis is much worse than hepatic and pulmonary metastases. CRC peritoneal metastasis is hard to diagnose early, presents with severe symptoms, and has a poor prognosis. It is crucial to emphasize standardized diagnosis and treatment for CRC peritoneal metastasis in order to improve patients' outcome and enhance their quality of life. This consensus, based on evidence-based medical evidence, revised the "Chinese expert consensus on the diagnosis and treatment of colorectal cancer peritoneal metastasis (2022 edition)", and reached a preliminary consensus on the definition, diagnosis, treatment, and
prevention of CRC peritoneal metastasis, with the aim of guiding and standardizing the diagnosis and treatment of CRC metastasis, developing reasonable and effective comprehensive treatment plans, prolonging survival time and improving the quality of life for CRC peritoneal metastasis patients, thereby raising the overall level of diagnosis and treatment for CRC in China.
Key words: colorectal neoplasms; peritoneal metastasis; diagnosis; treatment; consensus
Colorectal cancer is one of the most common malignant tumors, seriously threatening human health. Approximately 192.6118 million new cases of colorectal cancer are diagnosed worldwide each year, and approximately 90.3859 million people die from colorectal cancer [1]. Statistics released by the National Cancer Center in 2024 show that in 2022, there were 577,100 new cases of colorectal cancer in China, ranking second among malignant tumors, with an incidence rate of 20.1/100,000; and 240,000 deaths, a mortality rate of 8.56/100,000, ranking fourth [2]. Metastasis and recurrence are the main causes of death in colorectal cancer patients. The peritoneum is a common site of colorectal cancer metastasis, second only to liver and lung metastases, but the prognosis is much worse than that of liver and lung metastases.
Peritoneal metastasis of colorectal cancer refers to the shedding of cancer cells from the primary lesion of colorectal cancer directly and/or via blood vessels and lymphatic vessels, implanting on the peritoneum to form new lesions. 7% to 15% of colorectal cancer patients are diagnosed with peritoneal metastasis at initial diagnosis, and 4% to 19% of patients develop peritoneal metastasis after radical surgery [3-4]. Patients with peritoneal metastasis of colorectal cancer have a poor prognosis. If they do not receive active treatment after diagnosis, the median overall survival (OS) is only 6-9 months; the more severe the peritoneal metastasis, the worse the prognosis and the shorter the survival time [5-7]. Before the 1990s, peritoneal metastasis was considered the terminal stage of colorectal cancer treatment, and only palliative symptomatic treatment was usually adopted, while systemic chemotherapy had extremely poor efficacy due to the peritoneal barrier [5-7].
With the deepening of research, the development of new drugs, and the innovation and improvement of treatment technologies, patients with peritoneal metastasis of colorectal cancer can adopt active treatment methods, and the prognosis of some patients with lower tumor burden has been significantly improved. By evaluating the tumor burden of colorectal cancer peritoneal metastasis through the peritoneal cancer index (PCI), and then selecting cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC)/normothermic intraperitoneal chemotherapy (NIPEC)/systemic chemotherapy/targeted therapy and immunotherapy, etc., multidisciplinary comprehensive treatment is an active treatment method for colorectal cancer peritoneal metastasis.
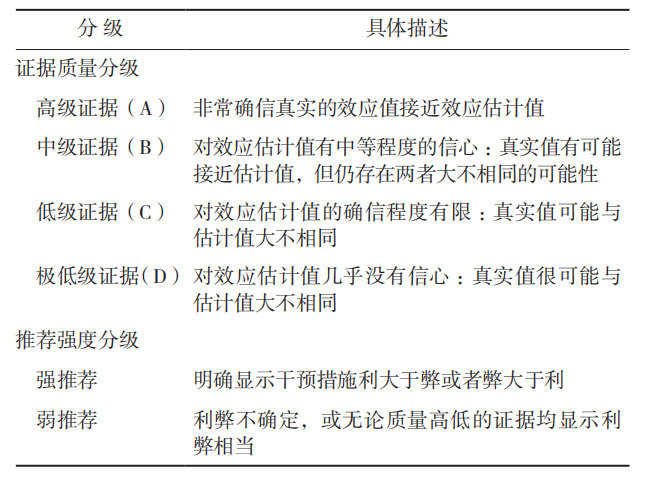
To standardize the diagnosis and treatment of colorectal cancer peritoneal metastasis, the Chinese Medical Doctor Association Colorectal Tumor Professional Committee organized domestic colorectal cancer experts to compile the "Chinese Expert Opinion on the Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2017 Edition)" [8] as early as 2017. In 2022, the "Chinese Expert Consensus on the Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2022 Edition)" [9] was revised and compiled. In view of the research progress on colorectal cancer peritoneal metastasis, the Chinese Medical Doctor Association Colorectal Tumor Professional Committee organized multidisciplinary experts to revise and update the 2022 version of the consensus. After repeated discussions and revisions, the "Chinese Expert Consensus on the Diagnosis and Treatment of Colorectal Cancer Peritoneal Metastasis (2025 Edition)" (hereinafter referred to as the Consensus) was compiled. This Consensus uses the Grade of Recommendation Assessment, Development and Evaluation (GRADE) grading standard to grade the quality of evidence and recommendations: the quality of evidence is divided into high-level evidence (A), intermediate-level evidence (B), low-level evidence (C), and very low-level evidence (D); the strength of recommendation is divided into strong recommendation and weak recommendation (Table 1) [10].
1. Definition of colorectal cancer peritoneal metastasis
Recommendation 1: Colorectal cancer peritoneal metastasis refers to the shedding and implantation of cancer cells from the primary lesion on the peritoneum, forming new tumor lesions. (Evidence quality: A; Recommendation level: Strong recommendation) The seed and soil theory was first proposed by Paget [11] in 1889,
and is considered the most important hypothetical theory for peritoneal metastasis [12]. This theory suggests that peritoneal metastasis is a multi-stage, multi-step process involving multiple factors, mainly including: (1) Cancer cells break through the serosal layer and shed into the abdominal cavity; (2) Cancer cells adhere to the peritoneum; (3) Cancer cells invade the peritoneum, promote the formation of surrounding neovascularization, and develop into metastatic lesions.
The sources of free cancer cells in the abdominal cavity mainly include three aspects: (1) Natural shedding from the serosal layer during tumor invasion and growth; (2) Shedding through blood vessels and/or lymphatic vessels and/or lymph nodes; (3) Iatrogenic dissemination during surgery, such as squeezing or pulling of the tumor during surgery can cause iatrogenic peritoneal implantation metastasis [11-12].
Table 1 Rating the quality of evidence by Grade of Recommendation Assessment, Development and Evaluation guidelines [10]
Table 1 Rating the quality of evidence by Grade of Recommendation Assessment, Development and Evaluation guidelines
Recommendation 2: Colorectal cancer with peritoneal metastasis at initial diagnosis is synchronous peritoneal metastasis, while peritoneal metastasis occurring after radical surgery is asynchronous peritoneal metastasis. Peritoneal metastasis diagnosed intraoperatively without preoperative imaging examination is defined as clinically occult peritoneal metastasis. (Evidence quality: A; Recommendation level: Strong recommendation) 7% to 15% of colorectal cancers have peritoneal metastasis at initial diagnosis, and 4% to 19% of patients develop peritoneal metastasis after radical surgery [3-4].
Thin-section enhanced CT is the most commonly used method for preoperative examination of peritoneal metastasis, with high sensitivity and specificity. However, 10% to 30% of patients are diagnosed as having no peritoneal metastasis by preoperative CT examination, but peritoneal metastasis is found during intraoperative exploration. This part of patients with peritoneal metastasis that is not found by imaging but is confirmed during intraoperative exploration is defined as clinically occult peritoneal metastasis [13-14].
2. Diagnosis of colorectal cancer peritoneal metastasis
The diagnosis of colorectal cancer peritoneal metastasis should combine medical history, clinical manifestations, imaging, tumor markers, and endoscopy. For suspicious patients, surgical exploration (laparoscopic exploration or laparotomy) can be used for diagnosis. Colorectal cancer peritoneal metastasis needs to be differentiated from peritoneal tuberculosis and sclerosing peritonitis.
Recommendation 3: Typical symptoms of colorectal cancer peritoneal metastasis are intractable ascites, persistent intestinal obstruction, and intractable abdominal pain. (Evidence quality: A; Recommendation level: Strong recommendation) In the early stages of colorectal cancer peritoneal metastasis, when the tumor burden is small, there are no obvious symptoms. When the tumor progresses to a certain extent and the tumor burden is large, typical symptoms of peritoneal cancer such as intractable ascites, persistent intestinal obstruction, and intractable abdominal pain may appear. The larger the tumor burden, the more severe these symptoms are.
Recommendation 4: Whole abdomen and pelvic thin-section enhanced CT is the preferred imaging modality for peritoneal metastasis of colorectal cancer. (Evidence quality: A; Recommendation level: Strong recommendation) The sensitivity of thin-section enhanced CT in diagnosing peritoneal metastasis of colorectal cancer is 25%~100%, and the specificity is 78%~100%. However, CT has certain limitations in diagnosing smaller peritoneal nodules. For peritoneal metastatic nodules with a diameter <5 mm, the sensitivity of CT diagnosis is only 11%~48%[13-15]. Typical CT findings of peritoneal metastasis of colorectal cancer include direct signs such as uneven thickening of the peritoneum with peritoneal nodules, multiple strands or nodules in the omentum or greater omentum, nodular thickening of the mesentery, and a large amount of ascites in the abdomen and pelvis, as well as indirect signs such as dilatation of the bile duct, ureter, and intestinal tract [15-17]. Multiplanar reconstruction of CT helps to clarify the location and distribution of peritoneal metastasis. Combined with CT findings, the preoperative CT-PCI score can be estimated to judge the tumor burden of peritoneal metastasis [15-17]. In addition to routine CT examination, PET/CT examination can also be considered, with sensitivity and specificity of 78%~97% and 55%~90%, respectively [17-19]. The detection efficiency of PET/CT depends on the uptake rate of 18F-fluorodeoxyglucose (18F-FDG) by cancer cells, which is related to the expression of glucose transporter-1 (GLUT1) [18-20]. Signet ring cell carcinoma, mucinous adenocarcinoma, and poorly differentiated adenocarcinoma are the most common histological types of peritoneal metastasis, while GLUT1 expression is extremely low in the above tissue types [19-20]. Using fibroblast activating protein inhibitor (FAPI) as a new metabolic imaging agent for PET/CT can detect some small peritoneal metastatic nodules, which is helpful in improving the diagnostic rate of peritoneal metastasis of colorectal cancer [20]. MRI can display the peritoneal structure under the premise of low-tension and respiratory training-controlled motion interference. It is recommended to use diffusion weighted imaging (DWI), which can more accurately assess the tumor burden, with sensitivity and specificity of 90% and 95.5%, respectively [21]. PET-MRI has the advantages of low radiation and high soft tissue resolution. It can analyze DWI and other magnetic resonance parameters simultaneously and combine the imaging results of PET probes, which is more advantageous than PET/CT [22].
In addition, gastrointestinal dynamic angiography can be performed to observe the intestinal motility and distribution, the time for the contrast agent to pass through each segment of the small intestine, and the condition of mesenteric retraction [23].
Recommendation 5: Serum tumor markers can be used as an auxiliary diagnostic tool for peritoneal metastasis of colorectal cancer. (Evidence quality: A; Recommendation level: Strong recommendation) Serum tumor marker detection plays an important auxiliary role in the diagnosis and treatment of peritoneal metastasis of colorectal cancer. The combined detection of carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), and CA19-9 can improve sensitivity and specificity [24-26]. CEA can help judge the degree of tumor invasion. CA19-9 can help judge the proliferation activity of cancer cells in ascites or primary lesions. CA125 can help judge the formation of ascites and the degree of tumor burden in peritoneal cancer, and the positive predictive value is higher than other markers [24-26]. In colorectal cancer patients with significantly elevated CEA, CA125, and CA19-9 without metastasis to other sites, peritoneal metastasis should be highly suspected. In colorectal cancer patients, persistent elevation of tumor markers during postoperative follow-up without metastasis or recurrence in other sites should also be highly suspected of peritoneal metastasis.
Recommendation 6: Surgical exploration (laparotomy or laparoscopy) is the most reliable method for diagnosing suspected peritoneal metastasis and assessing the extent of peritoneal metastasis, which helps in clinical treatment decision-making. (Evidence quality: A; Recommendation level: Strong recommendation) The detection rate of early peritoneal metastasis by routine imaging is low. Surgical exploration (laparoscopy or laparotomy) is the most reliable way to assess the tumor burden of peritoneal metastasis, especially for the detection of clinically occult peritoneal metastasis. Both sensitivity and specificity are high, and it can accurately assess the tumor burden of peritoneal metastasis, assisting in clinical treatment decision-making. At the same time, it can also perform peritoneal free cancer cell detection and peritoneal nodule biopsy to provide specimens for molecular detection [14].
Recommendation 7: PCI and peritoneal surface disease severity score (PSDSS) assess the tumor burden of peritoneal metastasis of colorectal cancer. (Evidence quality: A; Recommendation level: Strong recommendation) The tumor burden scoring systems for peritoneal metastasis mainly include PCI and PSDSS.
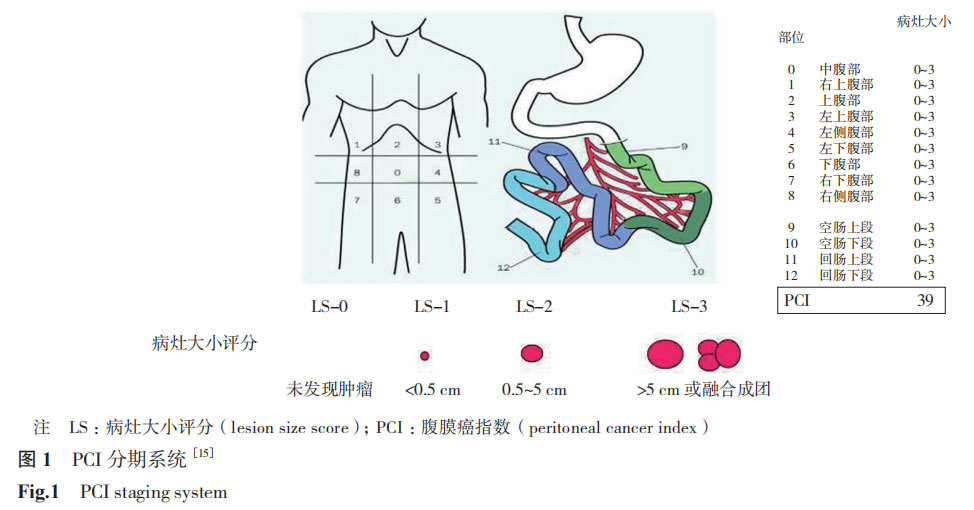
PCI is a scoring system for quantifying the tumor burden of peritoneal cancer, summarizing the size and distribution of tumor implants in 13 regions of the abdomen and pelvis, and quantifying the severity of peritoneal surface tumors [15,27].
The lesion size score (LS) is divided into 4 levels: LS-0, no tumor; LS-1, tumor <0.5 cm; LS-2, tumor 0.5~5 cm; LS-3, tumor >5 cm or fusion. The PCI score is the sum of the LS of each area, with a maximum of 39 points and a minimum of 0 points (no metastatic nodules visible to the naked eye, Figure 1) [15]. The PCI score is closely related to prognosis and is also an important indicator for CRS. The higher the PCI score, the lower the possibility of complete CRS surgery and the worse the prognosis.
PSDSS is a scoring system based on patient clinical symptoms, the extent of peritoneal dissemination, and the histopathological characteristics of the primary lesion (Table 2) [28].
According to the total score, PSDSS is staged: PSDSS Ⅰ, 2~3 points; PSDSS Ⅱ, 4~7 points; PSDSS Ⅲ, 8~10 points; PSDSS Ⅳ, >10 points. PSDSS is an independent prognostic factor for patients with peritoneal metastasis.
Table 2 PSDSS scoring standard [28]
Table 2 PSDSS evaluation criteria
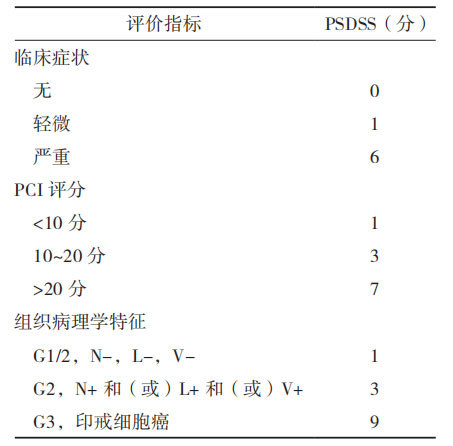
Note: PSDSS: peritoneal surface disease severity score; PCI: peritoneal cancer index; Clinical symptoms: mild is weight loss <10%, mild abdominal pain and a small amount of ascites, severe is weight loss ≥10%, persistent abdominal pain, intestinal obstruction and symptomatic ascites; PCI score: assessed by imaging examination or intraoperative assessment; N-/+: lymph node negative/positive; L-/+: lymphatic vessel invasion negative/positive; V-/+: vascular invasion negative/positive
Treatment of peritoneal metastasis of colorectal cancer
The treatment of peritoneal metastasis of colorectal cancer requires different multidisciplinary comprehensive treatment models according to the tumor burden, combining systemic and local treatments.
3.1 Systemic treatment
Recommendation 8: Systemic treatment refers to the treatment of metastatic colorectal cancer, including chemotherapy, targeted therapy, and immunotherapy.
(Evidence quality: A; Recommendation level: Strong recommendation) Systemic treatment includes chemotherapy, targeted therapy, immunotherapy, and symptomatic supportive treatment. For patients who can tolerate high-intensity treatment, mFOLFOX6, CAPEOX, FOLFIRI, or FOLFOXIRI, with or without targeted drugs or immunotherapy, are recommended [29].
3.1.1 Chemotherapy First-line and second-line chemotherapy regimens are as follows.
First-line chemotherapy regimens include mFOLFOX6, CAPEOX, FOLFIRI, and FOLFOXIRI regimens. (1) mFOLFOX6 (2 weeks/cycle): Oxaliplatin 85 mg/m² intravenous infusion for 2 h, d1; Calcium folinate 400 mg/m² intravenous infusion for 2 h, d1; Fluorouracil 400 mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total amount 2400 mg/m² continuous intravenous infusion for 46~48 h). (2) CAPEOX (3 weeks/cycle): Oxaliplatin 130 mg/m² intravenous infusion >2 h, d1; Capecitabine 1000 mg/m² oral, 2 times/d, d1~14. (3) FOLFIRI (2 weeks/cycle): Irinotecan 180 mg/m² intravenous infusion 60~90 min, d1; Calcium folinate 400 mg/m² intravenous infusion for 2 h (immediately after irinotecan infusion), d1; Fluorouracil 400 mg/m² intravenous bolus, d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total amount 2400 mg/m² continuous intravenous infusion for 46~48 h). (4) FOLFOXIRI (2 weeks/cycle): Irinotecan 165 mg/m² intravenous infusion, d1; Oxaliplatin 85 mg/m² intravenous infusion, d1; Calcium folinate 400 mg/m² intravenous infusion, d1; Fluorouracil total amount 2400~3200 mg/m², d1, continuous intravenous infusion for 48 h.
Second-line chemotherapy regimens include mFOLFOX6 or CAPEOX, FOLFIRI, oxaliplatin + raltitrexed (fluorouracil intolerant), and irinotecan + raltitrexed (fluorouracil intolerant).
(1) mFOLFOX6 (2 weeks/cycle) or CAPEOX (3 weeks/cycle): Specific chemotherapy dosage is the same as above, suitable for patients who failed irinotecan treatment in the first line. (2) FOLFIRI (2 weeks/cycle): Specific chemotherapy dosage is the same as above, suitable for patients who failed oxaliplatin treatment in the first line. (3) Oxaliplatin + raltitrexed (fluorouracil intolerant; 2 weeks/cycle): Oxaliplatin 85 mg/m² intravenous infusion for 2 h, d1; Raltitrexed 2 mg/m² intravenous infusion for 15 min, d1. (4) Irinotecan + raltitrexed (fluorouracil intolerant; 2 weeks/cycle): Irinotecan 180 mg/m² intravenous infusion for 60~90 min, d1; Raltitrexed 2 mg/m² intravenous infusion for 15 min, d1.
3.1.2 Targeted therapy First-line and second-line treatment regimens are as follows.
First-line treatment includes bevacizumab (trade name: Avastin) and cetuximab (trade name: Erbitux). (1) Bevacizumab:
Used for patients whose primary lesions are located in the right hemicolon, or whose primary lesions are located in the left hemicolon and are RAS or BRAF mutation types. There are two administration methods: 7.5 mg/kg intravenous infusion, d1 (3 weeks/cycle); 5 mg/kg intravenous infusion,
d1 (2 weeks/cycle). (2) Cetuximab: Used for patients whose primary lesions are located in the left hemicolon and are both RAS and BRAF wild-type. There are two administration methods: 400 mg/m²
initial intravenous infusion >2 h, subsequent 250 mg/m² intravenous infusion >60 min (1 week/cycle); 500 mg/m² intravenous infusion >2 h, d1 (2 weeks/cycle).
Second-line treatment includes bevacizumab and cetuximab. (1) Bevacizumab, treatment regimen and dosage are the same as before. Suitable for colorectal cancer patients who failed first-line treatment, regardless of RAS and BRAF phenotype, regardless of whether first-line treatment was combined with cetuximab or bevacizumab. (2) Cetuximab, treatment regimen and dosage are the same as before.
Only suitable for advanced colorectal cancer that did not combine cetuximab in the first-line treatment and is both RAS and BRAF wild-type.
3.1.3 Immunotherapy Microsatellite instability (MSI) and mismatch repair status (MMR) are the best predictors of immunotherapy efficacy.
Microsatellite stability status is divided into high MSI type (MSI-high, MSI-H), low MSI type (MSI-low, MSI-L), and microsatellite stability type (MSS). MMR is divided into deficient MMR (dMMR) and proficient MMR (pMMR). MSI-H colorectal cancer belongs to "hot tumors" and has good efficacy for immunotherapy. There is currently no high-level evidence-based medicine evidence for immunotherapy of MSI-L, MSS, and pMMR colorectal cancer.
3.2 Intraperitoneal chemotherapy
Recommendation 9: Commonly used intraperitoneal chemotherapy methods mainly include HIPEC and NIPEC. (Evidence quality: A; Recommendation level: Strong recommendation)
Due to the presence of the peritoneal-plasma barrier, high concentrations of chemotherapeutic drugs can be infused into the abdominal cavity to eliminate tumor cells without causing serious systemic toxicity. However, the passive penetration of intraperitoneal chemotherapeutic drugs is only 1-3 mm. Therefore, to improve the efficacy of local treatment, forms such as HIPEC and NIPEC can be adopted. HIPEC increases drug penetration by increasing temperature. NIPEC involves implanting a chemotherapy pump in the abdominal wall to infuse chemotherapeutic drugs at a fixed frequency and in multiple doses. It has advantages such as minimal trauma, long duration of action, flexibility, convenience, and outpatient use.
Recommendation 10: For HIPEC, a closed system is recommended, with an infusion rate of 400-600 mL/min, an effective infusion volume of 4-6 L, based on abdominal distension, and maintaining the intraperitoneal chemotherapeutic drug solution temperature at
(43.0±0.1) ℃ for 60-90 min. Postoperatively, multiple infusions can be performed according to the patient's specific condition, with each treatment interval being 24 h. (Evidence quality: A; Recommendation level: Strong recommendation)
In 1980, Spratt et al. [30] first reported the use of HIPEC to treat peritoneal malignant tumors. HIPEC refers to heating a chemotherapeutic drug-containing infusion solution to a therapeutic temperature of (43.0±0.1) ℃ and infusing it into the patient's abdominal cavity for a period of time. In recent years, technical methods have been continuously improved, gradually evolving into the current precise HIPEC technology. It has become a mature clinical application technology and has a unique therapeutic effect in the prevention and treatment of peritoneal tumors and associated malignant ascites. The principle of HIPEC is that cancer cells in a 43℃ environment, continuously soaked and flushed by liquid, can undergo irreversible damage. Normal tissues can tolerate 47℃ high temperature for 1 h. The difference in temperature tolerance between different tissues is used to perform targeted tumor killing at a specific temperature; the multiple thermal effects of HIPEC can lead to tumor vessel thrombosis, inhibit tumor angiogenesis, and disrupt tumor cell homeostasis, causing tumor cell degeneration and necrosis; heat therapy can enhance the toxicity of chemotherapeutic drugs to tumor cells, strengthening drug sensitivity and penetration; continuous peritoneal
lavage can physically flush free intraperitoneal cancer cells and small peritoneal lesions, removing residual intraperitoneal cancer cells and free lesions; heat shock proteins can be further activated under the effect of warmth, inducing anti-tumor immune effects, leading to tumor protein denaturation [29].
For HIPEC, a closed system is recommended. The closed method allows for repeated infusions postoperatively. The infusion rate is 400-600 mL/min, the effective infusion volume is 4-6 L, based on abdominal distension, and the intraperitoneal chemotherapeutic drug solution temperature is maintained at (43.0±0.1) ℃ for 60-90 min. Postoperatively, multiple infusions can be performed according to the patient's specific condition, with each treatment interval being 24 h.
Recommendation 11: Commonly used drugs for NIPEC include fluorouracil, oxaliplatin, and mitomycin. (Evidence quality: B; Recommendation level: Weak recommendation)
NIPEC involves direct injection of chemotherapeutic drugs into the abdominal cavity, bypassing the blood-peritoneal barrier, allowing the drugs to directly contact the lesions and exert their anti-tumor effects. For NIPEC, chemotherapeutic drugs should be selected based on the commonly used intravenous chemotherapeutic drugs for the primary tumor, previous sensitive drugs, or drug sensitivity test results; drugs with high tumor tissue penetration, large molecular weight, long half-life, low peritoneal absorption rate, synergistic effect with thermal effects, and low peritoneal irritation should be selected. For colorectal cancer peritoneal metastasis, NIPEC drugs include fluorouracil, oxaliplatin, mitomycin, irinotecan, raltitrexed, lobaplatin, cisplatin, and biological response modifiers (such as recombinant human tumor necrosis factor). Given that there are no unified standards for the dosage, types, and frequency of intraperitoneal drug use, the above drugs have significant differences in efficacy and adverse reactions. Therefore, they should be selected according to the patient's specific condition and with reference to the dosage of intravenous drugs; at the same time, almost none of the currently clinically used chemotherapeutic drugs have indications for NIPEC. Therefore, when used clinically, it is recommended that each medical center register all NIPEC drugs for off-label use, and sign an informed consent form for off-label drug use, and try to use anti-tumor drugs with systemic chemotherapy indications. It is recommended to conduct NIPEC drug clinical trials in centers with the necessary conditions to accumulate more data.
3.3 CRS
Recommendation 12: For colorectal cancer peritoneal metastasis with PCI ≤20, CRS can be considered, aiming for complete resection of all macroscopically visible intraperitoneal tumors to reduce tumor burden. (Evidence quality: A; Recommendation level: Strong recommendation)
CRS refers to the surgical removal of all macroscopically visible intraperitoneal tumors to reduce tumor burden, i.e., removal of all tumors from the parietal and visceral peritoneum, including involved organs and tissues, and dissection of relevant regional lymph nodes. The exploration and operation sequence of CRS is: round ligament of the liver, greater omentum, lesser omentum, right upper abdomen, left upper abdomen, diaphragmatic peritoneum, lateral wall peritoneum, right iliac fossa, left iliac fossa, pelvic floor peritoneum, and small intestinal mesentery. In addition, to ensure maximum tumor cell reduction, regional en bloc stripping of the parietal peritoneum is performed, and sites prone to tumor implantation, such as the gallbladder fossa, splenic fossa, and rectouterine pouch, are actively treated. Cholecystectomy, splenectomy, rectal resection, and removal of uterine appendages should be performed as appropriate [31-37]. Studies have shown that patients with PCI >20 have a worse prognosis after CRS than those with PCI ≤20, and the incidence of perioperative complications and surgery-related mortality are higher than those with PCI ≤20. Therefore, CRS should be considered cautiously in patients with PCI >20 [31-37]. When PCI ≤20, the decision of whether to perform CRS should be made in combination with gender, age, primary tumor stage, histological type, metastasis to other organs, ascites, extent of peritoneal metastasis, and surgeon's experience [31-37].
Recommendation 13: The completeness of cytoreduction (CC) score is used to assess the extent of residual tumor after CRS. (Evidence quality: A; Recommendation level: Strong recommendation)
In 1996, Jacquet et al. [27] proposed using the CC score to evaluate the extent of residual tumor after CRS. CC-0: No visible peritoneal nodules after CRS; CC-1: Residual tumor nodules <2.5 mm; CC-2: Residual tumor nodules 2.5 mm~2.5 cm; CC-3: Residual tumor nodules >2.5 cm, or residual unresectable tumor nodules or confluent lesions in the abdomen and pelvis. CC-2 and CC-3 are considered incomplete CRS.
Residual tumor nodules <2.5 mm (CC-0 and CC-1) are considered satisfactory CRS.
3.4 Multidisciplinary Comprehensive Treatment
Studies have shown that comprehensive treatment centered on CRS+HIPEC is more effective than systemic treatment alone in patients with peritoneal metastasis of colorectal cancer [38-44]; the median OS of patients receiving comprehensive treatment centered on CRS+HIPEC reached 22.3 months, while the median OS of patients receiving chemotherapy alone was only 12.6 months. The 5-year OS rate of patients who achieved CC-0 or CC-1 after CRS reached 45% [38-39].
Recommendation 14: Treatment of peritoneal metastasis of colorectal cancer should
Select a comprehensive treatment regimen combining systemic treatment, NIPEC, and CRS based on tumor burden (PCI score). (Evidence quality: A; Recommendation level: Strong recommendation)
For patients with peritoneal metastasis of colorectal cancer at initial diagnosis or recurrence after treatment, surgical exploration (laparotomy or laparoscopy) is recommended for accurate PCI scoring. When PCI>20, a comprehensive treatment regimen of systemic treatment + NIPEC can be used for conversion therapy. If PCI is reduced to ≤20 after treatment, the decision of whether to perform CRS should be made based on the patient's gender, age, primary tumor stage, histological type, metastasis to other organs, ascites, extent of peritoneal metastasis, and surgeon's experience [35-37]. Even if PCI cannot be reduced to ≤20 to achieve the goal of CRS, it can still be used as palliative treatment to improve the patient's quality of life and prolong survival time. When PCI≤20, a comprehensive treatment regimen of CRS+NIPEC+ systemic treatment can be adopted [35-37]. For patients with low PCI scores where CRS can achieve CC-0 and CC-1, there is no high-level evidence to confirm that neoadjuvant therapy can improve survival rate and prolong survival time [45]. The possibility of recurrence after CRS is very high, even with complete CRS, therefore, postoperative treatment, including systemic treatment and intraperitoneal chemotherapy, must be emphasized [46-48].
4 Prevention of Peritoneal Metastasis of Colorectal Cancer
Recommendation 15: High-risk factors for peritoneal metastasis of colorectal cancer include perforation of the primary colorectal cancer lesion, concomitant ovarian metastasis, non-R0 resection of the primary lesion, TNM stage T4 and (or) N+, less than 12 lymph nodes collected during surgery, poorly differentiated histological type (such as mucinous adenocarcinoma), and positive cytology for free cancer cells in peritoneal lavage fluid. (Evidence quality: A; Recommendation level: Strong recommendation)
High-risk factors for the development of peritoneal metastasis of colorectal cancer include [47-49]: (1) Perforation of the primary colorectal cancer lesion; (2) Concomitant ovarian metastasis; (3) Non-R0 resection of the primary lesion; (4) TNM stage T4 and (or) N+; (5) Less than 12 lymph nodes collected during surgery; (6) Positive cytology for free cancer cells in peritoneal lavage fluid; (7) Low tumor differentiation grade, such as mucinous adenocarcinoma. In addition, young age at onset, tumor invasion of intestinal wall nerves, and emergency surgery are also risk factors for peritoneal metastasis of colorectal cancer.
Recommendation 16: Standardized comprehensive treatment of locally advanced colorectal cancer, strict en bloc resection during surgery, and prevention of iatrogenic peritoneal dissemination are effective means of preventing peritoneal metastasis. (Evidence quality: A; Recommendation level: Strong recommendation)
For patients with high-risk factors for peritoneal metastasis, a series of preventive measures should be taken to reduce the incidence of peritoneal metastasis. The main measures include: (1) Standardized perioperative treatment for patients with locally advanced colorectal cancer can reduce the risk of peritoneal metastasis. Treatment plans can be selectively implemented after discussion by a multidisciplinary team (MDT); (2) Strictly adhere to the principle of en bloc resection during surgery to minimize the shedding and implantation of cancer cells into the peritoneal cavity due to surgical manipulation, preventing iatrogenic dissemination; (3) Although there is still a lack of high-level evidence on the role of prophylactic HIPEC in preventing peritoneal metastasis of colorectal cancer, studies have shown that as part of comprehensive treatment, HIPEC can reduce the incidence of peritoneal metastasis and improve survival rate and prolong survival time in colorectal cancer patients with high-risk factors for peritoneal metastasis [50-56]. Therefore, it is recommended that prophylactic HIPEC treatment be selectively performed in the form of clinical research in units with the necessary conditions to accumulate more clinical evidence.
Team Leader: Xiong Bin (Zhongnan Hospital of Wuhan University), Wang Xishan (Cancer Hospital, Chinese Academy of Medical Sciences), Wang Hui (Sixth Affiliated Hospital of Sun Yat-sen University), Yuan Weitang (First Affiliated Hospital of Zhengzhou University), Cui Shuzhong (Affiliated Cancer Hospital of Guangzhou Medical University)
Writers: Xiong Bin (Zhongnan Hospital of Wuhan University), Pei Wei (Cancer Hospital, Chinese Academy of Medical Sciences), Yang Chaogang (Zhongnan Hospital of Wuhan University)
Peer Review Experts (in alphabetical order by pinyin): Bu Jianhong (Editorial Department of Chinese Journal of Gastrointestinal Surgery), Cai Guoxiang (Fudan University Cancer Hospital), Cai Jian (The Second People's Hospital of Shenzhen), Cui Shuzhong (Affiliated Cancer Hospital of Guangzhou Medical University), Deng Haijun (Southern Medical University Southern Hospital), Ding Kefeng (The Second Affiliated Hospital of Zhejiang University School of Medicine), Han Fanghai (The Second People's Hospital of Guangdong Province), Han Shuai (Zhujiang Hospital of Southern Medical University), Hu Junhong (First Affiliated Hospital of Zhengzhou University)
Huang Chaoqun (Zhongnan Hospital of Wuhan University), Leng Jiahua (Peking University Cancer Hospital), Li Yong (Guangdong Provincial People's Hospital), Lian Yugui (First Affiliated Hospital of Zhengzhou University), Lu Yiping (Beijing University of Chinese Medicine Affiliated Hospital), Pei Haiping (Xiangya Hospital, Central South University), Pei Wei (Cancer Hospital, Chinese Academy of Medical Sciences), Peng Zheng (The First Medical Center of PLA General Hospital), Shen Zhanlong (Peking University People's Hospital), Su Guoqiang (Affiliated Hospital of Xiamen University)
First Hospital), Sun Lifeng (The Second Affiliated Hospital of Zhejiang University School of Medicine), Sun Xuejun (The First Affiliated Hospital of Xi'an Jiaotong University), Tang Hongsheng (Affiliated Tumor Hospital of Guangzhou Medical University), Wang Guiying (Hebei Medical University / The Second Hospital of Hebei Medical University), Wang Huiming (The Sixth Affiliated Hospital of Sun Yat-sen University), Wang Hui (The Sixth Affiliated Hospital of Sun Yat-sen University), Wang Xishan (Cancer Hospital, Chinese Academy of Medical Sciences), Wang Xiaoqiang (Shaanxi Provincial People's Hospital), Wang Zhenning (China Medical University / The First Affiliated Hospital of China Medical University), Wang Ziwei (The First Affiliated Hospital of Chongqing Medical University), Wu Wei (Xiangya Hospital, Central South University), Wu Aiwen (Peking University Cancer Hospital), Xiong Bin (Zhongnan Hospital of Wuhan University), Yang Chaogang (Zhongnan Hospital of Wuhan University), Yang Xiaojun (Zhongnan Hospital of Wuhan University), Yao Hongliang (The Second Xiangya Hospital of Central South University), Yuan Weitang (The First Affiliated Hospital of Zhengzhou University), Yuan Ying (The Second Affiliated Hospital of Zhejiang University School of Medicine), Zhang Xuan (Yunnan Provincial Tumor Hospital), Zhang Zilong (Jingzhou Central Hospital), Zheng Jianbao (The First Affiliated Hospital of Xi'an Jiaotong University), Zhou Baojun (The Second People's Hospital of Hebei Province), Zhu Yuping (Zhejiang Cancer Hospital)
Conflict of Interest All experts involved in writing and reviewing the discussion declared
No conflict of interest
References:
[1] Bray F, Laversanne M, Sung H, et al. Global cancer sta tistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263.
[2] Chinese Anti-Cancer Association. China Tumor Integrated Diagnosis and Treatment Guidelines (Second Edition) Lower Volume [M]. Tianjin: Tianjin Science and Technology Press, 2024: 1527.
[3] Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal car cinomatosis of colorectal origin: incidence and current treatment strategies[J]. Ann Surg, 2006, 243(2): 212-222.
[4] Klaver YL, Lemmens VE, Nienhuijs SW, et al. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options[J]. World J Gastroenterol, 2012, 18(39): 5489-5494.
[5] Franko J, Shi Q, Goldman CD, et al. Treatment of colorec atal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase Ⅲ trials N9741 and N9841[J]. J Clin Oncol, 2012,30(3): 263-267.
[6] Foster JM, Zhang C, Rehman S, et al. The contemporary management of peritoneal metastasis: a journey from the cold past of treatment futility to a warm present and a bright future[J]. CA Cancer J Clin, 2023, 73(1): 49-71.
[7] Lemmens VE, Klaver YL, Verwaal VJ, et al. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study[J]. Int J Can cer, 2011, 128(11): 2717-2725.
[8] Colorectal Tumor Professional Committee of the Chinese Medical Doctor Association Peritoneal Tumor Professional Committee. Chinese Expert Opinion on the Diagnosis and Treatment of Peritoneal Metastasis of Colorectal Cancer (2017)[J]. Chinese Journal of Colorectal Diseases, 2017, 6(5): 360-366.
[9] Colorectal Tumor Professional Committee of the Chinese Medical Doctor Association Peritoneal Tumor Professional Committee. Chinese Expert Consensus on the Diagnosis and Treatment of Peritoneal Metastasis of Colorectal Cancer (2022 Edition)[J]. Chinese Journal of Colorectal Diseases, 2022, 11(4): 265-271.
[10] Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence[J]. J Clin Epidemiol, 2011, 64(4): 401-406.
[11] Paget S. The distribution of secondary growths in cancer of the breast. 1889[J]. Cancer Metastasis Rev, 1989, 8(2): 98-101.
[12] Pietrasik JM, Uruski P, Tykarski A, et al. The peritoneal "soil" for a cancerous "seed": a comprehensive review of the pathogenesis of intraperitoneal cancer metastases[J]. Cell Mol Life Sci, 2018, 75(3): 509-525.
[13] Kim SJ, Kim HH, Kim YH, et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients un dergoing surgery for gastric cancer[J]. Radiology, 2009, 253(2): 407-415.
[14] Dong D, Tang L, Li ZY, et al. Development and validation of an individualized nomogram to identify occult peritone atal metastasis in patients with advanced gastric cancer[J]. Ann Oncol, 2019, 30(3): 431-438.
[15] Koh JL, Yan TD, Glenn D, et al. Evaluation of preopera tive computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis[J]. Ann Surg Oncol, 2009, 16(2): 327-333.
[16] Wang J, Hu Y, Xiong H, et al. CT-based deep learning model: a novel approach to the preoperative staging in pa tients with peritoneal metastasis[J]. Clin Exp Metastasis, 2023, 40(6): 493-504.
[17] Dromain C, Leboulleux S, Auperin A, et al. Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT[J]. Abdom Imaging, 2008, 33(1): 87-93.
[18] Yamada A, Oguchi K, Fukushima M, et al. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transport er-1 expression[J]. Ann Nucl Med, 2006, 20(9): 597-604.
[19] Shimada H, Okazumi S, Koyama M, et al. Japanese Gas tric Cancer Association Task Force for Research Promo tion: clinical utility of 18F-fluoro-2-deoxyglucose positron emission tomography in gastric cancer. A systematic re view of the literature[J]. Gastric Cancer, 2011, 14(1): 13-21.
[20] Zhao L, Pang Y, Luo Z, et al. Role of [68Ga] Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [18F]-FDG PET/CT[J]. Eur J Nucl Med Mol Imaging, 2021, 48(6): 1944-1955.
[21] Zhang H, Dai W, Fu C, et al. Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy[J].
Cancer Biol Med, 2018, 15(2): 165-170.
[22] Qin C, Shao F, Gai Y, et al. 68Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with 18F-FDG PET/CT[J]. J Nucl Med, 2022, 63(1): 81-
88.
[23] Mei LJ, Wang LW, Huang CQ, et al. Oral gastrografin radiography for the evaluation of the functional impact of peritoneal carcinomatosis: correlation with clinicopathological findings[J]. Mol Clin Oncol, 2015, 3(5): 979-986.
[24] Yang XQ, Chen C, Peng CW, et al. Carbohydrate antigen 242 highly consists with carbohydrate antigen 19-9 in diagnosis and prognosis of colorectal cancer: study on 185 cases[J]. Med Oncol, 2012, 29(2): 1030-1036.
[25] Chen C, Chen LQ, Yang GL, et al. The application of C12 biochip in the diagnosis and monitoring of colorectal cancer: systematic evaluation and suggestion for improvement[J]. J Postgrad Med, 2008, 54(3): 186-190.
[26] Wagner PL, Austin F, Sathaiah M, et al. Significance of serum tumor marker levels in peritoneal carcinomatosis of appendiceal origin[J]. Ann Surg Oncol, 2013, 20(2): 506-514.
[27] Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis[J]. Cancer Treat Res, 1996, 82: 359-374.
[28] Pelz JO, Stojadinovic A, Nissan A, et al. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis[J]. J Surg Oncol, 2009, 99(1): 9-15.
[29] 中国抗癌协会. 中国肿瘤整合诊治指南(第二版)下[M]. 天津 : 天津科学技术出版社, 2024: 1671-1673.
[30] Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy[J]. Cancer Res, 1980, 40(2): 256-260.
[31] Sugarbaker PH. Peritonectomy procedures[J]. Surg Oncol Clin N Am, 2003, 12(3): 703-727, xiii.
[32] Yonemura Y, Elnemr A, Endou Y, et al. Surgical results of patients with peritoneal carcinomatosis treated with cytoreductive surgery using a new technique named aqua dissection[J]. Gastroenterol Res Pract, 2012, 2012: 21487.
[33] Mizumoto A, Canbay E, Hirano M, et al. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan[J]. Gastroenterol Res Pract, 2012, 2012: 836425.
[34] Huang CQ, Yang XJ, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase Ⅱ study from a Chinese center [J]. PLoS One, 2014, 9(9): e108509.
[35] Sugarbaker PH. Cytoreductive surgery plus hyperthermic perioperative chemotherapy for selected patients with peritoneal metastases from colorectal cancer: a new standard of care or an experimental approach?[J]. Gastroenterol Res Pract, 2012, 2012: 309417.
[36] Elias D, Mariani A, Cloutier AS, et al. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin[J]. Eur J Surg Oncol, 2014, 40(11): 1467-1473.
[37] Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study[J]. J Clin Oncol, 2004, 22(16): 3284-3292.
[38] Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer[J]. J Clin Oncol, 2003, 21(20):3737-3743.
[39] Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer[J]. Ann Surg Oncol, 2008, 15(9): 2426-2432.
[40] Huang CQ, Feng JP, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center[J]. J Surg Oncol, 2014, 109(7): 730-739.
[41] Franko J, Ibrahim Z, Gusani NJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis[J]. Cancer, 2010, 116(16): 3756-3762.
[42] Mirnezami R, Mehta AM, Chandrakumaran K, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone[J]. Br J Cancer, 2014, 111(8): 1500-1508.
Taqi K, Lee J, Hurton S, et al. Long-term outcomes following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin[J]. Curr Oncol, 2024, 31(7): 3657-3668.
Kitaguchi D, Park EJ, Baik SH, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus R0 resection for resectable colorectal cancer with peritoneal metastases and low peritoneal cancer index scores: a collaborative observational study from Korea and Japan[J]. Int J Surg, 2024, 110(1): 45-52.
Sarofim M, Wijayawardana R, Ahmadi N, et al. Neoadjuvant chemotherapy does not improve survival for patients with high volume colorectal peritoneal metastases undergoing cytoreductive surgery[J]. World J Surg Oncol, 2024, 22(1): 103-109.
Chen D, Ma Y, Li J, et al. Risk factors for postoperative complications in patients undergoing cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: A meta-analysis and systematic review[J]. Int J Colorectal Dis, 2024, 39(1): 167-183.
Dietz MV, Hannink G, Said I, et al. Development of a prediction model for recurrence in patients with colorectal peritoneal metastases undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy[J]. Eur J Surg Oncol, 2024, 50(6): 108294.
Tonello M, Cenzi C, Pizzolato E, et al. Systemic chemotherapy in colorectal peritoneal metastases treated with cytoreductive surgery: systematic review and meta-analysis[J]. Cancers, 2024, 16(6): 1182-1197.
Sammartino P, Sibio S, Biacchi D, et al. Long-term results after proactive management for locoregional control in patients with colonic cancer at high risk of peritoneal metastases[J]. Int J Colorectal Dis, 2014, 29(9): 1081-1089.
Cortes-Guiral D, Elias D, Cascales-Campos PA, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy for patients with colorectal cancer at
high risk of peritoneal carcinomatosis: Does it really save lives?[J]. World J Gastroenterol, 2017, 23(3): 377-381.
Segelman J, Akre O, Gustafsson UO, et al. External validation of models predicting the individual risk of metachronous peritoneal carcinomatosis from colon and rectal cancer[J]. Colorectal Dis, 2016, 18(4): 378-385.
Baratti D, Kusamura S, Iusco D, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) at the time of primary curative surgery in patients with colorectal cancer at high risk for metachronous peritoneal metastases[J]. Ann Surg Oncol, 2017, 24(1): 167-175.
Zheng Y, Zhang J, Chen C, et al. Prophylactic hyperthermic intraperitoneal chemotherapy in T4 colorectal cancer: Can it improve the oncologic prognosis?-A propensity score matching study[J]. Eur J Surg Oncol, 2024, 50(2): 107958.
Zhou H, Wang H, Yi S, et al. Effectiveness of hyperthermic intraperitoneal chemotherapy during primary curative resection for colorectal carcinoma[J]. Int J Colorectal Dis, 2024, 39(1): 197.
Sun BJ, Daniel SK, Lee B. The role of prophylactic and adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention of peritoneal metastases in advanced colorectal cancer[J]. J Clin Med, 2023, 12(20): 6443.
Arjona-Sánchez A, Espinosa-Redondo E, Gutiérrez-Calvo A, et al. Efficacy and safety of intraoperative hyperthermic intraperitoneal chemotherapy for locally advanced colon cancer: a phase 3 randomized clinical trial[J]. JAMA Surg, 2023, 158(7): 683-691.
Manuscript received: 2025-01-22