CACA China Integrated Diagnosis and Treatment Guidelines for Peritoneal Tumors - 2025 Edition
Peritoneal tumors are mainly classified as primary and secondary. Primary tumors originate from the peritoneum; common types include primary peritoneal cancer (Müllerian-type epithelial tumors, mainly serous carcinoma) and malignant peritoneal mesothelioma (MPM). Secondary tumors include various metastatic cancers, peritoneal sarcomatosis (PS), pseudomyxoma peritonei (PMP), and gliomatosis. Clinically, metastatic and epithelial malignant peritoneal tumors are more common, while primary and mesenchymal tumors are relatively rare and considered rare diseases.
Release time:
2025-07-10
Source:
Honorary Editor-in-Chief
Dai-ming Fan
Editor-in-Chief
Shuzhong Cui, Zhenggang Zhu, Ximo Wang, Kaixiong Tao, Han Liang, Zhongqiu Lin
Associate Editor-in-Chief
Kefeng Ding, Xiaoqing Jiang, Yan Li, Zhenning Wang, Jiankun Hu, Bin Xiong, Guoxiang Cai, Zheng Peng
Qun Zhao, Gang Ji, Hongsheng Tang
Editorial Board Members (in alphabetical order by pinyin surname)
Jianyong Ao, Mingchen Ba, Xuebin Bao, Xiaogang Bi, Guoxiang Cai, Jiaqing Cao, Jie Chai, Huanqiu Chen
Xiaolei Chen, Shuzhong Cui, Maojun Di, Kefeng Ding, Jiangtao Fan, Runya Fang, Xuedong Fang, Feiling Feng
Yuming Fu, Yunong Gao, Peiming Guo, Mian He, Xianli He, Li Hong, Mingxing Hou, Jiankun Hu
Wenqing Hu, Yingbin Hu, Chen Huang, Guangjian Huang, Yong Ji, Gang Ji, Xiaoqing Jiang, Zhigang Jie
Changqing Jing, Ziying Lei, Bo Li, Feng Li, Gang Li, Jing Li, Sang Li, Yan Li
Hongyu Li, Jiansheng Li, Jundong Li, Qiling Li, Yongxiang Li, Yuzhi Li, Yunfeng Li, Bin Liang
Han Liang, Wei Liang, Zhongqiu Lin, Dechun Liu, Jianhua Liu, Naifu Liu, Wentao Liu, Yefu Liu
Ning Lu, Jiali Luo, Puyin Luo, Jun Ma, Hongchao Mou, Ke Pan, Minghui Pang, Zhigang Pang
Wei Pei, Haiping Pei, Zheng Peng, Peng Qu, Xingan Qin, Yizhou Qin, Zhen Shen, Yan Shi
Weidong Shi, Jun Song, Zhan Song, Hao Sun, Li Sun, Jianhua Sun, Lifeng Sun, Fengbo Tan
Xiaodong Tan, Hongsheng Tang, Kaixiong Tao, Yantao Tian, Biao Wang, Xuefei Wang, Dong Wang, Jin Wang
Jing Wang, Ke Wang, Kuan Wang, Li Wang, Ning Wang, Quan Wang, Wei Wang, Daorong Wang
Guangwei Wang, Guihua Wang, Jinghan Wang, Shufeng Wang, Ximo Wang, Xiaozhong Wang, Yubin Wang, Zhenning Wang
Shoujiang Wei, Zhenping Wen, Chuanqing Wu, Xiaomei Wu, Yinbing Wu, Xiaohong Xia, Yabin Xia, Bin Xiong
Zekuan Xu, Zhiyuan Xu, Tianmin Xu, Min Xue, Chao Yan, Zhilong Yan, Zhen Yang, Zhuo Yang
Jianjun Yang, Xianzi Yang, Desheng Yao, Feng Ye, Jianxin Ye, Weimin Yi, Lanning Yin, Qingchen Yin
Weiming Yu, Yayi Yuan, Yujian Zeng, Hui Zhang, Guonan Zhang, Huifeng Zhang, Jiangyu Zhang, Mingsheng Zhang
Xiangliang Zhang, Yujing Zhang, Gang Zhao, Ping Zhao, Qun Zhao, Chunlin Zhao, Xiaoning Zhao, Xi Zhong
Yanbing Zhou, Linghua Zhu, Zhenggang Zhu
Authors
Ziying Lei, Xianzi Yang, Jiangyu Zhang, Yayi Yuan, Hongsheng Tang, Jing Li, Xi Zhong, Jianyong Ao
Secretary
Ziying Lei, Jiali Luo
Chapter 1 Overview of Peritoneal Tumors
Peritoneal tumors have a poor overall prognosis. In the past, due to limited medical conditions, the detection rate was low. With the continuous improvement of diagnostic and therapeutic techniques and the continuous improvement of pathological diagnosis level, the number of confirmed cases has been increasing year by year, and it has received more and more attention from the medical community.
Section 1 Classification of Peritoneal Tumors
Peritoneal tumors are mainly divided into primary and secondary types. Primary tumors are tumors originating from the peritoneum, commonly including primary peritoneal cancer (i.e., Mullerian-type epithelial tumors, mainly serous carcinomas) and malignant peritoneal mesothelioma (MPM). Secondary tumors include various metastatic cancers, peritoneal sarcomatosis (PS), pseudomyxoma peritonei (PMP), and gliomatosis. Clinically, metastatic and epithelial-derived malignant peritoneal tumors are more common, while primary and mesenchymal-derived tumors are relatively rare and are considered a rare disease.
(1) Primary peritoneal tumors
This mainly refers to malignant tumors originating from the secondary Mullerian duct or peritoneal mesothelium, which grow multifocally. Primary peritoneal cancer, i.e., Mullerian-type epithelial tumors, is relatively rare. The classic histological feature is serous carcinoma (SC), which is divided into high-grade and low-grade types, consistent with the same type of tumor with the same degree of differentiation originating from the ovary. During surgery, bilateral ovaries are observed to be of normal size, or physiologically enlarged, or enlarged due to benign diseases, or only superficially involved, and no primary ovarian tumor is found. MPM is a rare primary malignant tumor originating from peritoneal mesothelial cells. It can occur in the parietal or visceral peritoneum, with diffuse or localized distribution. It can invade abdominal and pelvic organs, and can also implant on the surface of abdominal and pelvic organs and metastasize to other organs through lymph or blood vessels.
(2) Secondary peritoneal tumors
It usually refers to the direct shedding and implantation growth of cancer cells from the primary lesion, or metastasis via hematogenous/lymphatic peritoneal routes. It is clinically common and often secondary to tumors in the stomach, colon, rectum, ovary, appendix, hepatobiliary system, pancreas, uterus, and retroperitoneum. It can also be secondary to tumors in the lung, breast, brain, bone, nasopharynx, and skin melanoma. Peritoneal metastasis of malignant tumors in the abdomen and pelvis, such as gastric cancer, colorectal cancer, ovarian cancer, and appendiceal mucinous tumors, is relatively common.
Approximately 20% of patients with advanced gastric cancer have peritoneal metastasis at initial diagnosis, and 50% develop peritoneal metastasis after radical surgery. In advanced colorectal cancer, 7% to 15% have peritoneal metastasis at initial diagnosis, and 4% to 19% develop peritoneal metastasis after radical surgery. Among these, the peritoneal metastasis rate after T4 stage surgery is 20% to 36.7%. Approximately 75% of patients with ovarian cancer have peritoneal metastasis at initial diagnosis.
PMP is mainly caused by the rupture of mucin-secreting tumors, leading to the accumulation and redistribution of a large amount of mucinous ascites in the abdominal cavity. It mainly involves the diaphragmatic peritoneum and greater omentum, with approximately 90% originating from the appendix and belonging to low-grade malignant mucinous tumors.
Section 2: Pathogenesis of Peritoneal Tumors
1. Pathogenesis of Primary Peritoneal Tumors
(1) Primary Peritoneal Carcinoma
The currently accepted theory is the Secondary Mullerian System (SMS) theory. Embryonic cells can differentiate into female abdominal serosal and Mullerian epithelial cells. Abdominal serosal and Mullerian epithelial cells have homology. Analysis of histological characteristics and tumor antigenicity further shows that female Mullerian tumors and peritoneal tumors have certain commonalities. In addition, the Mullerian duct is independent of sex during fetal development, and this disease is not limited to females; males can also develop it, but the incidence is much lower than in females.
(2) MPM
The incidence is often associated with asbestos; approximately 90% of MPM patients have a history of asbestos exposure, with a latency period of 25 to 70 years. Asbestos enters the body through the respiratory or digestive system and gradually accumulates in the peritoneum to form asbestos bodies, acting on target cells or inducing reactive oxygen free radicals, causing chromosomal aberrations, and ultimately leading to tumorigenesis. The occurrence of MPM is also influenced by genetic factors to some extent and is the result of the interaction between environmental carcinogens and genetic susceptibility. Current carcinogenic factors include chemical carcinogens such as asbestos and other mineral fibers, and physical carcinogens such as chronic peritonitis and therapeutic radiation. Other physical and chemical carcinogens include zeolite, talc, mica, and xylene.
2. Pathogenesis of Secondary Peritoneal Tumors
Secondary peritoneal tumors are peritoneal metastases of various tumors. Their core mechanism conforms to the classic "seed and soil" theory. Cancer cells are the "seeds," often free cancer cells (FCCs) that are released from tumor tissue before or during surgery. The seeds often play a decisive role; the microenvironment of the peritoneum is the "soil," composed of growth factors released to promote wound healing after peritoneal injury during surgery, inflammatory cells, blood residues, blood clots, exposed mesothelial tissue, and fibrin deposition. Cancer cells easily implant in this environment. Due to the lack of a continuous layer of mesothelial cells, cancer cells easily colonize the specific structures of the peritoneum—lymphatic stomata and lacunae. Peritoneal metastasis of tumors is a complex process that can be roughly divided into three steps:
(1) Tumor cell shedding or release to form metastatic foci
Gastric cancer and ovarian cancer are the most common, followed by colorectal cancer, pancreatic cancer, gallbladder cancer, liver cancer, and endometrial cancer. Lung cancer and breast cancer can also metastasize to the peritoneum.
Intraperitoneal tumor metastasis is often due to the rapid growth of the primary tumor, local invasion and penetration of the serosal tissue on the surface of the organ, shedding into the abdominal cavity, and the formation of multiple metastatic foci in the peritoneum.
Cancer cells that are not properly isolated during surgery and fall into the gastrointestinal tract flow into the abdominal cavity with gastrointestinal fluid through the residual end. Cancer emboli in blood vessels and lymphatic vessels that are severed in the surgical area flow into the abdominal cavity with blood and lymph. Cancer cells in the abdominal cavity are solidified by fibrinous material in the surgical area, forming a protective layer that makes them less susceptible to killing by immune cells, forming residual small cancer foci. Coupled with surgery and anesthesia, the body's immunity is reduced, cancer cells proliferate to form masses, eventually leading to local recurrence and metastasis in the abdominal cavity.
The above two situations are the main causes of secondary peritoneal tumors. Clinically, peritoneal metastatic tumors of unknown origin are also seen, and the primary lesion is difficult to identify even after various examinations.
(2) Cancer cells or nests spread in the abdominal cavity
Any factor that reduces the closed volume of the abdominal cavity will increase intra-abdominal pressure, leading to the shedding and dissemination of cancer cells or nests to various parts of the abdominal cavity. It is currently believed that tumor cells undergo a series of biological changes during peritoneal metastasis and spread, which helps their survival in ascites and peritoneal invasion. Cancer cells can separate from the primary tumor and enter the abdominal cavity as single cells or multicellular tumor spheroids (MTCS). Compared with single cancer cells, MTCS can overcome the anoikis of single cancer cells, and their migration and invasion abilities are significantly enhanced. These biological MTCS characteristics can significantly promote cancer cell growth and metastasis and are adaptive changes that occur in tumor cells to survive at the metastatic site. The formation of MTCS is related to multiple factors. Angiotensin II (Ang II) can significantly improve the formation, growth, and invasion ability of ovarian cancer cell line MTCS and promote peritoneal metastasis, mainly by directly activating the mitogen-activated protein kinase (MAPK)/extracellular regulated protein kinase (ERK) pathway and through the epidermal growth factor receptor (EGFR).
(3) Cancer cells or nests colonize the peritoneum
Cancer cells or nests that have detached and spread to the abdominal cavity adhere to the peritoneum, stimulating inflammation. The adhesion molecules produced by the latter further promote the "rooting" of cancer cells. Cancer-associated fibroblasts (CAFs) can induce cancer cells in ascites to surround them, forming a special MTCS. CAFs are located in the center of the MTCS and promote cancer cell proliferation, peritoneal adhesion, and invasion by secreting epidermal growth factor (EGF). Although the copy number alterations (CNAs) and single nucleotide variants (SNVs) of MTCS differ from those of the primary lesion, they still reflect 92.3%~100.0% of the mutations in the primary tumor, indicating a high degree of homology between the attached MTCS and the abdominal serosa.
Metastatic cancer cells secrete TGF-β, which directly and indirectly acts on endothelial cells to promote angiogenesis and migration in the tumor microenvironment (TME), stimulate extracellular matrix (ECM) deposition, and alter the TME. Various innate and adaptive immune cells are scattered among them. TGF-β can inhibit the immune system by modulating the function of immune cell populations in the peritoneal tumor node TME, helping metastatic cancer cells inhibit the adaptive immunity of tumors by inhibiting the activation, proliferation, differentiation, and migration of T cells. It can also block the activation and maturation of cytotoxic CD8+ T cells by inhibiting the presentation of tumor antigens and the expression of DCs, and inhibit the proliferation of CD8+ T cells by inhibiting the expression of IFN-γ and IL-2. TGF-β can promote the expression of programmed cell death protein 1 (PD-1) induced by antigens in CD8+ T cells, leading to T cell exhaustion, resulting in immune escape of metastatic cancer cells and achieving peritoneal colonization and adhesion.
Section 3: Clinical Manifestations of Peritoneal Tumors
(1) Primary peritoneal tumors
It progresses insidiously, with early occult symptoms and no obvious symptoms. At a certain stage, it is discovered. Patients may have abdominal distension, abdominal pain, ascites, abdominal masses, and other changes, and may also be accompanied by anorexia, oliguria, constipation, weight loss, intestinal obstruction, cachexia, etc.
(2) Secondary peritoneal tumors
It mainly occurs secondary to gastric cancer, colorectal cancer, ovarian cancer, appendiceal mucinous tumors, etc., and generally has a long course. Diagnosis is made by integrating the history of the primary tumor, signs, imaging evidence, and pathological results. Patients diagnosed with peritoneal metastasis have a worse condition than general tumor patients. Some patients, due to a heavier tumor burden, present with fatigue, emaciation, cachexia, anemia, and other consumptive signs, manifesting as depression. Different secondary peritoneal tumors have different clinical manifestations due to different primary tumors, but there are also similarities, mainly manifested as abdominal masses, abdominal distension, ascites, ureter/renal pelvis dilatation, rectal or bladder irritation symptoms, digestive system and systemic symptoms, etc.
Section 4: Diagnosis of Peritoneal Tumors
Regardless of whether it is primary or secondary peritoneal tumor, the clinical manifestations lack specificity. Various imaging examinations such as ultrasound, CT, MRI, and PET/CT provide references. Laparoscopic exploration and laparotomy are widely used in the diagnosis of peritoneal tumors, while cytology, histopathology, and immunohistochemistry play a key role in the diagnosis of the origin and pathological type of peritoneal tumors.
Section 5: Current Status of Peritoneal Tumor Treatment
Peritoneal tumors are numerous, difficult to treat, and have poor efficacy. Their treatment has been a concern in academia for a long time, but the efficacy has not been broken through, and the intractable ascites, abdominal pain, intestinal obstruction, and other complications caused by this disease have not achieved satisfactory efficacy. The traditional view is that peritoneal tumors belong to the terminal stage of tumors, with a short survival period, only 3-6 months, and only palliative symptomatic treatment is needed.
Since the late 20th century, with the continuous updating of the consensus on peritoneal tumors, after more than 40 years of research by oncologists, a new treatment concept of cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been explored. CRS can maximally remove organs and serosa involved by tumors, and HIPEC can remove and control small residual tumor tissues and free cancer cells through thermal therapy, chemotherapy, synergistic thermal chemotherapy, and mechanical flushing, which can significantly improve the integrated efficacy of peritoneal tumors. CRS+HIPEC has shown significant efficacy in preventing and treating the planting and dissemination, recurrence and metastasis of malignant tumors in the abdomen and pelvis, improving survival rate and quality of life, and has been widely promoted in clinical practice.
Chapter 2: Prevention and Screening of Peritoneal Tumors
Section 1: Prevention of Peritoneal Tumors
1. Prevention of Primary Peritoneal Tumors
The etiology of primary peritoneal tumors is not yet fully understood. Primary prevention is etiological prevention, including smoking control and alcohol limitation, reducing or avoiding contact with carcinogens (including physical, chemical, and biological factors); advocating a reasonable diet and good exercise habits, maintaining good health. Secondary prevention is early diagnosis and treatment, screening for tumors in high-risk populations, early detection of primary peritoneal tumor patients, and early diagnosis and treatment. Tertiary prevention is integrated treatment, palliative symptomatic treatment, combining appropriate treatment strategies according to the patient's condition, actively preventing complications, reducing the damage of tumors to the body, and improving the prognosis.
2. Prevention of Secondary Peritoneal Tumors
Gastric cancer, colorectal cancer, ovarian cancer, appendiceal mucinous tumors, hepatobiliary pancreatic cancer, etc., can spontaneously shed FCCs during disease progression, or shed tumor cells during surgery, which is the pathological basis for peritoneal metastasis. Removal of FCCs after surgery can reduce the incidence of peritoneal metastasis.
(1) Primary Prevention
This mainly refers to the active treatment of the primary disease. The primary cancer focus needs to be completely resected to achieve R0 resection. Strict adherence to the principle of no tumor cells is required for standardized operation, paying attention to incision protection, avoiding compression of the tumor, minimizing iatrogenic dissemination, and thoroughly cleaning the surrounding lymph nodes.
HIPEC can effectively remove FCCs, kill subclinical lesions that cannot be removed by surgery, reduce postoperative peritoneal metastasis and disease recurrence. The specific selection of perfusion chemotherapy drugs and solvents should be adjusted according to the type of primary tumor and drug sensitivity to achieve better preventive effects. Many results show that HIPEC has a significant therapeutic effect on controlling peritoneal metastasis recurrence after radical surgery. Many domestic and foreign prospective randomized controlled phase III clinical trials are underway (NCT02614534, NCT04370925, NCT02179489).
(2) Secondary prevention
This mainly refers to regular follow-up and check-ups after surgical resection of primary malignant tumors in the abdomen and pelvis, performing tumor marker and related imaging examinations, early detection of peritoneal metastasis, and timely integrated treatment mainly based on CRS+HIPEC treatment.
(3) Tertiary prevention
This mainly refers to the relevant treatment for advanced patients. These patients have many complications (peritoneal effusion, intestinal obstruction, cachexia, etc.), obvious cancerous pain, and require active clinical symptomatic supportive treatment to improve their quality of life.
Section Two: Screening for Peritoneal Tumors
1. Screening content for peritoneal tumors (see Table 29-2-1)
Table 29-2-1 Screening content for peritoneal tumors
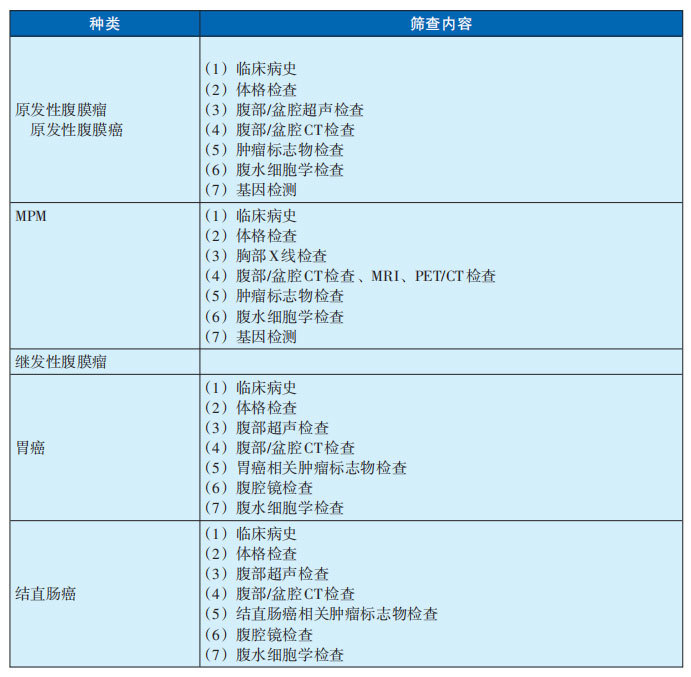
2. Screening recommendations for different populations
2.1 Screening for general-risk populations
Primary peritoneal tumors have a low incidence in clinical practice, early signs are not obvious, and diagnosis is difficult. They are often diagnosed in the middle and late stages of the disease. Peritoneal tumor screening is not recommended for the general population, but for people with a history of exposure to physical and chemical carcinogens, screening is recommended, including an ultrasound examination once a year and a CT scan if necessary.
For general-risk populations with secondary peritoneal tumors, it is recommended to follow the routine screening for each primary tumor. In the first two years after surgery, once every three months; then once every six months until the fifth year; and once a year after five years. This includes tumor markers, abdominal ultrasound, and CT scans.
2.2 Screening for high-risk populations
High-risk populations refer to those exposed to high-risk environments and are considered a key population for peritoneal tumor screening. To detect primary peritoneal tumors as early as possible, it is recommended that high-risk populations undergo screening once every six months. For high-risk populations with peritoneal metastasis, it is recommended that they undergo screening once every three months for the first three years after surgery, and then once every six months until the fifth year. This includes abdominal ultrasound and enhanced CT, CA125, CA199, CEA, and other related tumor marker tests.
(1) High-risk factors for primary peritoneal tumors
High-risk factors for primary peritoneal cancer:
The histology and clinical manifestations of primary peritoneal cancer are similar to those of ovarian cancer, and they are discussed together with ovarian cancer peritoneal metastasis.
① Family history. ② BRCA1/BRCA2 gene mutation. ③ History of pelvic radiotherapy. ④ >60 years old.
High-risk factors for MPM:
① History of asbestos dust exposure. ② Family history.
(2) High-risk factors for secondary peritoneal tumors
High-risk factors for gastric cancer secondary peritoneal metastasis:
① T3, T4 stage tumors. ② Positive test for free cancer cells in peritoneal lavage fluid. ③ Signet ring cell carcinoma. ④ Lymph node metastasis. ⑤ Borrmann type III, IV. ⑥ Lauren histological type is diffuse type. ⑦ Tumor perforation or rupture. ⑧ Accompanied by vascular/lymphatic vessel emboli, nerve invasion.
High-risk factors for colorectal cancer secondary peritoneal metastasis:
① T3, T4 stage tumors. ② Positive test for free cancer cells in peritoneal lavage fluid. ③ Tumor perforation or rupture. ④ Tumor causing intestinal obstruction. ⑤ Positive resection margin. ⑥ Lymph node metastasis or incomplete lymph node dissection (insufficient number of nodes dissected, less than 12). ⑦ Mucinous adenocarcinoma or signet ring cell carcinoma. ⑧ Accompanied by vascular/lymphatic vessel emboli, nerve invasion.
High-risk factors for appendiceal mucinous tumor peritoneal metastasis:
① Rupture of appendiceal mucinous tumor. ② Low tumor differentiation. ③ Insufficient surgical resection range.
Chapter Three: Diagnosis of Peritoneal Tumors
Section One: Diagnosis of Primary Peritoneal Tumors
Primary peritoneal tumors progress insidiously, with no obvious symptoms in the early stages, and are only discovered when the condition progresses to a certain stage. Patients may have abdominal changes such as abdominal distension, abdominal pain, peritoneal effusion, and abdominal masses. They may also have clinical manifestations such as anorexia, oliguria, constipation, weight loss, intestinal obstruction, and cachexia. Abnormal tumor markers combined with imaging examination results can lead to a preliminary diagnosis. To further clarify the pathological type, the most commonly used method is tumor puncture biopsy guided by ultrasound or CT. If there is ascites, a less invasive cytological examination of peritoneal effusion can be used. However, a tissue biopsy must still be performed under laparoscopic or open exploration for confirmation, depending on the clinical situation.
1. Clinical manifestations
1.1 Symptoms of primary peritoneal cancer (see Table 29-3-1)
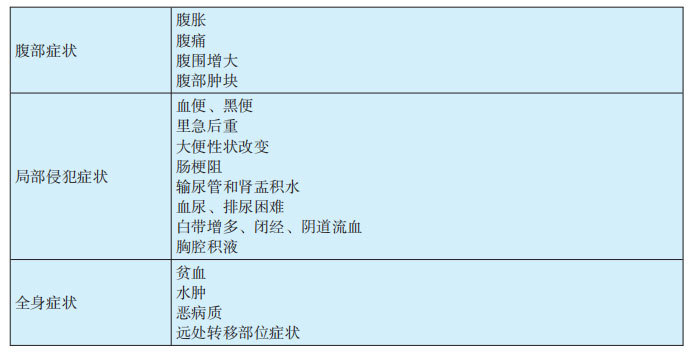
(1) Early symptoms are not obvious, and signs may be absent. Symptoms only appear when the abdominal tumor develops to a certain size and affects other important organs. There are three typical symptoms: ① Abdominal distension: Often the first symptom. When the tumor grows to a certain extent and compresses the intestines, or when peritoneal effusion reaches a certain amount, it can cause a feeling of abdominal fullness. The time and degree depend on the patient's subjective feeling and sensitivity. ② Abdominal pain: Initially, the abdomen experiences dull pain, aching pain, etc. When the tumor grows to cause severe intestinal obstruction or compression of the urethra, causing difficulty urinating, it manifests as abdominal colic or severe pain. ③ Increased abdominal circumference: As the tumor grows and ascites increases, the abdominal circumference gradually increases. After the tumor grows to a certain extent, an abdominal mass can be palpated.
(2) Tumor invasion of the colon can cause symptoms such as hematochezia, melena, tenesmus, and changes in stool characteristics. Enlargement of the tumor can cause severe intestinal obstruction, similar to the symptoms of colon cancer; compression of the ureter can cause hydronephrosis and pyelectasis; invasion of the bladder can cause hematuria; compression of the urethra can cause dysuria; in women, local invasion of bilateral adnexa can cause increased vaginal discharge, amenorrhea, and vaginal bleeding; when the tumor breaks through the abdominal cavity and invades the chest cavity, it can cause pleural effusion. Untreated patients or those who progress to the late stage of the disease may develop distant metastases to the lungs, brain, and liver, and experience corresponding symptoms.
(3) There are often non-specific systemic symptoms, which may be accompanied by varying degrees of anemia and edema. Some patients, due to disease progression, exhibit a cachectic constitution, with symptoms such as weight loss and low-grade fever.
1.2 Symptoms of MPM (see Table 29-3-2)
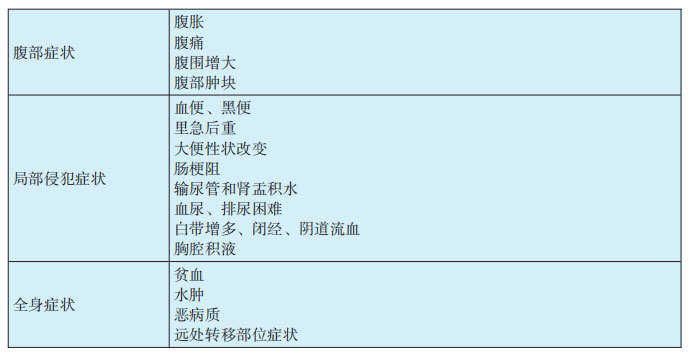
(1) MPM often has no specific manifestations. Common symptoms include abdominal distension, abdominal pain, ascites, and abdominal masses. These include: ① Abdominal pain: In the early stages, there is often no fixed location, and its occurrence is related to factors such as tumor involvement of surrounding tissues and organs, peritoneal irritation by ascites, and pain from traction of abdominal masses. Mild symptoms may be dull pain or stabbing pain. Severe pain can be paroxysmal colic or sudden severe pain, often located in the upper abdomen, but also in the lower abdomen or even during bowel movements. ② Abdominal distension: This is often related to ascites and abdominal masses, and in severe cases, it can cause dyspnea. Patients often have yellow exudative ascites or bloody viscous ascites. ③ Abdominal masses: This is one of the common clinical manifestations, which can be single or multiple, varying in size, and palpable as nodules, hard in texture. Pelvic masses can be detected by rectal examination or bimanual examination.
(2) Tumor compression of the gastrointestinal tract and intestinal adhesion can cause symptoms of intestinal obstruction. Patients often have symptoms such as poor appetite, nausea, fatigue, vomiting, constipation, and weight loss. MPM can involve various organs throughout the body through direct invasion, lymphatic system, or hematogenous metastasis, such as the abdominal wall, liver, gallbladder, pancreas, urinary system, heart, lungs, adrenal glands, bone marrow, and lymphatic system, and present with corresponding clinical manifestations.
2. Diagnostic methods for primary peritoneal tumors
2.1 Laboratory tests
Serological examination: CA125 is elevated in most patients with primary peritoneal carcinoma and MPM.
Ascites examination:
Detecting CA125 levels in ascites has a certain diagnostic value. When abdominal masses are found and solid lesions of the ovaries are ruled out, significantly elevated CA125 levels in ascites often suggest the possibility of primary peritoneal carcinoma and MPM. The level of CA125 is correlated with the clinical extent of the lesion; the wider the lesion, the higher the CA125 value.
Elevated CA125 is more common in ovarian cancer, but it can also be seen in tuberculosis, cervical cancer, peritoneal metastatic cancer, pancreatic cancer, gastric cancer, colon cancer, breast cancer, and endometriosis. Therefore, primary peritoneal carcinoma and MPM should be differentiated from peritoneal tuberculosis. The tumor CA125 value is generally significantly higher than that in tuberculosis; the CA125 value in peritoneal tuberculosis is generally not higher than 50 ng/L. Tuberculosis bacillus detection in ascites can confirm the diagnosis of peritoneal tuberculosis. A single CA125 test has low specificity in the diagnosis of primary peritoneal carcinoma and MPM and is of little significance for differential diagnosis; it can only provide a reference.
2.2 Imaging examinations
(1) Ultrasound
This is a commonly used examination method for primary peritoneal tumors. Typical signs include: ① Ascites: Fluid-filled dark areas are seen in the abdomen and pelvis, with floating and moving intestines. ② "Cake-like" greater omentum: The greater omentum is invaded and contracted, appearing as a cake-like or mass-like shadow. ③ Abdominal and pelvic wall nodules/masses: Medium/high-echo nodules or masses are seen on the surface of the intestines, peritoneum, and mesentery without obvious blood flow signals. ④ Enlarged lymph nodes: These are mostly adjacent to the primary cancer lesion, also seen in the root of the mesentery or retroperitoneum, appearing as nodular hypoechoic lesions with a diameter greater than 1 cm; when there are many lymph nodes and they are large, they can merge and easily undergo necrosis.
(2) CT
CT examination has the advantages of universality, speed, volumetric scanning, and multi-planar reconstruction. Typical CT findings of primary peritoneal tumors include: ① Ascites: Water-like density shadows are seen in the abdomen and pelvis; if there is bleeding, high-density or layered phenomena may appear. ② Greater omentum invasion and contraction: Increased fat density of the omentum, blurred boundaries, multiple millet-like nodules, and even a "omental cake" sign. ③ Abdominal and pelvic cavity, peritoneal solid nodules/masses: These are often multiple soft tissue density lesions, and enhanced scanning shows varying degrees of enhancement. ④ Enlarged lymph nodes: Soft tissue nodules with a diameter greater than 1 cm, with clearer boundaries after enhancement, and the solid component shows mild-to-moderate enhancement. Larger lymph nodes are prone to necrosis, and the necrotic area shows no enhancement. However, CT has a low detection rate for micronodules <2 mm in diameter; using thin-slice CT reconstruction can help improve the detection rate of small lesions.
(3) MRI
Compared with CT, MRI can improve the sensitivity of diagnosis of primary peritoneal tumors, especially with the application of MRI diffusion-weighted imaging (DWI), which provides a non-invasive method for evaluating the benign or malignant nature of tumors. In solid primary peritoneal tumors, T1WI shows low signal, T2WI shows slightly high signal, and DWI signal shows iso- or high signal (malignant tumors are mostly high signal, benign tumors are mostly iso-signal). Enhancement on T1WI shows obvious enhancement of the lesion; when the tumor undergoes cystic necrosis, T2WI shows significantly high signal, DWI shows low signal, and T1WI enhancement shows no enhancement in the area of cystic necrosis, but the cyst wall may enhance. However, MRI has a low detection rate for lesions <5 mm. A negative MRI cannot completely rule out primary peritoneal tumors.
(4) PET/CT
PET/CT detects differences in the uptake of fludeoxyglucose (FDG) by tissues to distinguish between benign and malignant lesions and their invasiveness.
Compared with conventional CT, PET/CT can improve diagnostic sensitivity and specificity, and its role in the differential diagnosis of primary peritoneal tumors is more prominent. However, PET/CT is expensive, equipment is scarce, and limitations such as isotope radiation and low soft tissue resolution restrict its use as a routine screening tool; and there is a certain "false positive" rate, with some benign tumors with high metabolic activity and inflammatory lymph nodes also showing high FDG uptake. Therefore, it is generally used as an alternative examination item when CT/MRI examination cannot meet the diagnostic requirements.
2.3 Pathological Examination
(1) Biopsy methods for primary peritoneal tumors
1) Detection of tumor cells in ascites
When there are few cancer cells, it is difficult to distinguish them from other tumor cells. The sensitivity of ascites cytology is often not high, but it can be distinguished from most non-tumor diseases. It has the advantages of high specificity, economy, simplicity, and speed, and is often used as the first-choice examination. Ascites is extracted by counter-McBurney's point puncture or laparoscopic puncture for ascites cytology examination to find cancer cells, and multiple tests can be performed if necessary. Ascites can also be centrifuged, the sediment embedded, and cell paraffin blocks made for HE staining observation and diagnosis. Immunohistochemistry, FISH detection, and next-generation sequencing can also help with diagnosis, differential diagnosis, and treatment guidance, but next-generation sequencing is not the first choice for guiding treatment in principle, and tissue samples should be tested first.
2) Peritoneal biopsy
It has decisive diagnostic significance for primary peritoneal tumors. It is usually divided into laparoscopy-assisted pathological biopsy or laparotomy biopsy. Compared with other examination methods, biopsy is more intuitive and accurate, and is the most direct basis for diagnosis. During laparoscopy or laparotomy, the nature, distribution, shape, size, and texture of lesions/nodules/masses can be intuitively understood, and ascites can be directly aspirated for detection and diagnosis. However, it is an invasive examination and is generally not the first choice.
Laparoscopic exploration has the advantages of minimal invasiveness and rapid recovery. Taking a biopsy under laparoscopic assistance can also intuitively and comprehensively assess the abdominal cavity situation and judge whether CRS can be performed laparoscopically or by laparotomy. It can also determine whether chemotherapy should be performed first before formulating the next treatment plan.
Laparotomy biopsy can intuitively understand the abdominal cavity situation. Intraoperative biopsy can directly perform maximum CRS, such as resection of digestive tract, uterus, ovaries, omentum, mesentery, and appendix lesions. If the abdominal cavity adhesion is severe, laparotomy also has greater flexibility. However, laparotomy has the disadvantages of excessive trauma and slow postoperative recovery.
(2) Pathological characteristics of primary peritoneal tumors
1) Primary peritoneal carcinoma
That is, peritoneal serous carcinoma, similar to low-grade or high-grade serous carcinoma of the ovary. It is mostly high-grade carcinoma, and the clinical and pathological characteristics are significantly different from low-grade carcinoma. High-grade carcinoma is more likely to occur in female patients with a median age of 62 years. The average age of onset of low-grade carcinoma is 52 years. TP53 and BRCA mutations are common in high-grade carcinomas, while KRAS and BRAF mutations are rare. High-grade carcinoma should be considered as one of the phenotypes of familial breast and ovarian cancer syndrome. Conversely, low-grade carcinoma often has KRAS and BRAF mutations but lacks TP53 mutations and BRCA abnormalities.
Low-grade carcinoma is equivalent to invasive implantation from borderline/atypical proliferative serous tumors, but more extensive, commonly associated with unique small nest tumor-like serous cells similar to low-grade serous carcinoma of the ovary. High-grade peritoneal serous carcinoma is similar to high-grade peritoneal serous carcinoma of the ovary.
The main basis for distinguishing high-grade peritoneal serous carcinoma from low-grade carcinoma is cellular atypia. Low-grade carcinoma has small and uniform nuclei, with less cellular atypia and rare mitotic figures. High mitotic activity tends to diagnose high-grade carcinoma. Tumor staging, treatment, and prognosis are similar to ovarian serous carcinoma. Low-grade carcinoma rarely progresses to high-grade tumors. Compared with high-grade carcinoma, low-grade carcinoma is insensitive to chemotherapy. Surgery is a more effective treatment method, and high-grade carcinoma can be treated in reference to similar tumors of the ovary and fallopian tube.
2) MPM
MPM is a highly malignant tumor, generally divided into biphasic malignant mesothelioma, epithelioid malignant mesothelioma, and sarcomatoid malignant mesothelioma.
① Biphasic malignant mesothelioma
It has both malignant epithelial and sarcomatous components, with each subtype accounting for at least 10%. The histology is similar to that of biphasic synovial sarcoma. The malignant epithelial component often presents as glandular, papillary, or cleft-like structures. Focal bone and cartilage metaplasia is occasionally seen in the spindle cell area, and occasionally scattered or focally distributed small round undifferentiated cells are seen. There is a transition between spindle cells and epithelial cells. Histochemistry and immunohistochemistry are very helpful in determining the diagnosis and differential diagnosis of mesothelioma. Histochemical PAS, AB, colloidal iron, etc., staining tumor cells are positive, and reticular fibers are positive between spindle cells and negative between epithelial cells.
② Epithelioid malignant mesothelioma
The tumor tissue of epithelioid malignant mesothelioma is mainly arranged in tubules, acini, and papillae, and some are also arranged in nests, cords, sheets, clefts, microcysts, or networks. The tumor cells are cuboidal or flattened, with abundant cytoplasm, red staining, or vacuolated, transparent, signet ring cell-like. The cytoplasm of some cells is filled with red-stained substances, forming hyaline bodies, PAS positive. The nuclei of tumor cells vary in size, with high atypia and many mitotic figures.
Decidual variant mesothelioma is a rare variant of epithelioid mesothelioma, more common in the abdominal cavity of young women, and is highly invasive. Microscopically, it is composed of large round or polygonal epithelioid or histiocytoid cells with abundant, eosinophilic, ground-glass-like cytoplasm, clear cell boundaries, vacuolated nuclei, and prominent eosinophilic nucleoli, similar to decidual cells during pregnancy. Cells show mild to moderate atypia, with rare mitotic figures, and locally visible rhabdomyoblast-like morphology.
③ Sarcomatoid malignant mesothelioma
The tumor cells are composed of spindle-shaped fibroblastic cells arranged in cords or haphazardly, very similar to fibrosarcoma.
Typical mesothelioma components are visible. In some cases, the atypia of tumor cells is obvious, mitotic figures are easily visible, and multinucleated tumor giant cells can be seen. Tumor cells can be arranged in a sheet-like pattern, similar to high-grade pleomorphic undifferentiated sarcoma. Some cases may show areas similar to leiomyosarcoma, osteosarcoma, chondrosarcoma, or other sarcomas, but the lesion is small. If the above lesions are extensive, it is easy to be confused with the above sarcomas.
The immunohistochemical characteristics of the above three types of malignant mesothelioma are shown in Table 29-3-3. The differentiation between MPM and serous carcinoma is often difficult and requires immunohistochemistry for differentiation. The differential indicators are shown in Table 29-3-4.
Table 29-3-3 Immunohistochemical characteristics of MPM
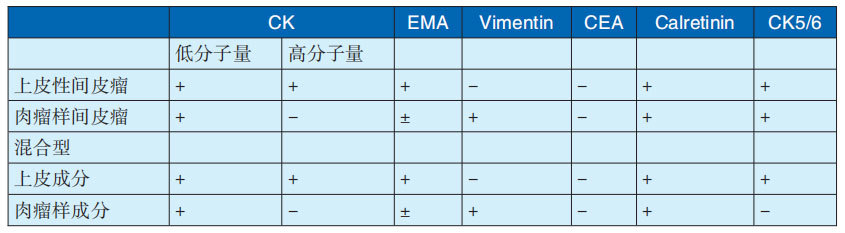
Note: Cited from Liu Tonghua, chief editor, "Liu Tonghua Diagnostic Pathology", 4th edition
Table 29-3-4 Immunohistochemical differentiation between MPM and serous carcinoma
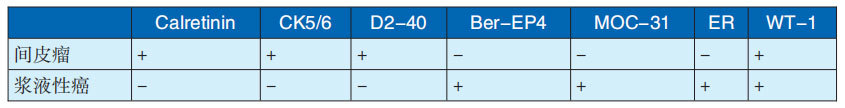
Note: Cited from Liu Tonghua, chief editor, "Liu Tonghua Diagnostic Pathology", 4th edition
3. Diagnosis and differential diagnosis
3.1 Diagnostic criteria for primary peritoneal tumors
(1) Diagnostic criteria for primary peritoneal carcinoma
The diagnostic criteria for primary peritoneal carcinoma generally use the American Gynecologic Oncology Group (GOG) criteria, mainly based on the volume of ovarian lesions and the depth of tumor infiltration:
① Both ovaries are consistent with normal physiological size, or only benign lesions are seen. ② The volume of bilateral ovarian lesions is smaller than that of extra-ovarian lesions. ③ Microscopic examination of ovarian lesions shows one of the following: A. No ovarian lesions are found. B. Tumor nodules are limited to the ovarian surface, and no stromal infiltration is found. C. Ovarian surface and stroma are involved, and the area of stromal involvement is less than 5mm × 5mm. D. Histological and cytological characteristics are mainly serous, similar to or the same as ovarian serous papillary adenocarcinoma, with varying degrees of differentiation.
(2) Diagnostic criteria for MPM
Patients with symptoms and signs such as abdominal distension, abdominal pain, abdominal mass, ascites, and weight loss, and CT or MRI showing diffuse omental mass, mesenteric nodules or nodular masses, and diffuse or localized thickening of the peritoneum, should be highly suspected of MPM.
The diagnosis is mainly based on: ① Symptoms: Patients who present clinically with abdominal pain, abdominal distension, ascites, and abdominal masses, especially those with a history of asbestos exposure. ② Imaging diagnosis: B-ultrasound, CT, MRI, PET/CT and other imaging evidence supporting the diagnosis of MPM. ③ Ascites detection: Tumor cells detected in ascites/peritoneal lavage fluid cytology. Significantly elevated CEA in ascites can rule out malignant mesothelioma, while abnormally elevated hyaluronic acid concentration supports the diagnosis of malignant mesothelioma. ④ Pathological examination: Puncture biopsy, laparoscopic or open surgical direct visualization biopsy supporting the diagnosis of MPM. ⑤ Exclusion of secondary peritoneal tumors.
3.2 Differential diagnosis of primary peritoneal tumors
(1) Tuberculous peritonitis
It is common in young and middle-aged women, and some may find evidence of pulmonary or extrapulmonary tuberculosis. The clinical manifestations of tuberculous peritonitis are low-grade fever, night sweats, abdominal pain, abdominal distension, ascites, and abdominal masses, which are difficult to differentiate from primary peritoneal tumors with non-specific clinical manifestations. Adenosine deaminase (ADA) in the ascites of tuberculous peritonitis can be detected as higher than normal values, and the detection of Mycobacterium tuberculosis in ascites culture can also confirm the diagnosis. A strongly positive tuberculin skin test or T-SPOT test supports the diagnosis of tuberculous peritonitis. CA125 in tuberculous peritonitis may be slightly elevated, but not as significantly as in primary peritoneal tumors, which is helpful in differentiating tuberculous peritonitis. Clinically, diagnostic treatment can be performed for patients with unclear diagnosis but high suspicion of tuberculous peritonitis. For those who are ineffective in treatment and cannot be diagnosed clearly, laparoscopic exploration and pathological biopsy can be performed for confirmation.
(2) Ascites due to cirrhosis
In the decompensated stage of cirrhosis, ascites increases, and there may be abdominal distension, abdominal discomfort, and abdominal distension, which need to be differentiated from peritoneal tumors with ascites. Ascites due to cirrhosis is closely related to portal hypertension and hepatic dysfunction. Ultrasound, CT, and MRI can all detect changes in liver morphology and splenomegaly, and laboratory tests can detect abnormal liver function. Ascites due to cirrhosis is mostly transudate, while peritoneal tumors are mostly exudate. Cancer cells found in ascites can rule out ascites due to cirrhosis.
(3) Peritonitis
Acute peritonitis often presents with severe abdominal pain, reflex nausea and vomiting, and systemic toxic symptoms. Physical examination shows total abdominal tenderness and peritoneal irritation signs, elevated white blood cells and neutrophils, and effective anti-infection treatment. Secondary peritonitis is more common and can be caused by trauma or organ perforation and rupture. CT helps to differentiate peritonitis from peritoneal tumors, and peritoneal puncture can help with diagnosis.
In primary peritonitis, there is no primary lesion in the abdominal organs. Among them, spontaneous peritonitis caused by decompensated cirrhosis is more common, and often presents with non-specific symptoms such as abdominal pain and distension, and liver function is often reduced. Diagnostic puncture shows elevated white blood cells in ascites, and pathogenic bacteria can be cultured, but the positive rate is not high.
(4) Ovarian cancer peritoneal metastasis
In primary peritoneal tumors, there is no primary lesion in the bilateral ovarian parenchyma, while ovarian cancer peritoneal metastasis can find ovarian cancer lesions while finding peritoneal tumors. Because the histological types of the two diseases are similar or even the same, immunohistochemistry is not very meaningful for their differentiation.
(5) Appendiceal mucinous tumor
Appendiceal mucinous tumor, more common in middle-aged men, is a low-grade malignant tumor. Mucin-secreting cells in the tumor break through the appendiceal wall and enter the abdominal cavity, implanting in the abdominal cavity to form PMPs. Early stages are often asymptomatic, with some patients presenting with abdominal mass as the only chief complaint. After PMP formation, complications such as mucinous ascites, abdominal distension, and cake-like omentum may occur. When significant abdominal distension occurs, abdominal inspection shows that the abdominal shape is not like a "frog belly", and percussion reveals no shifting dullness. Abdominal paracentesis often fails to extract ascites, but a thicker needle can extract gelatinous viscous fluid. Ultrasound examination has high specificity, showing a large amount of flocculent echoes in the abdominal cavity, with slow movement of light spots, light spots, and light rings in the dark area.
Section 2: Diagnosis of Secondary Peritoneal Tumors
The diagnosis of secondary peritoneal tumors is mainly based on the integrated diagnosis of the history of primary tumors, clinical signs, imaging evidence of peritoneal metastasis, and pathological examination results. Clinical manifestations lack specificity. Ultrasound, CT, MRI, and PET/CT imaging examinations can only play a reference role in the preoperative diagnosis of the extent, degree, and tumor burden of lesions. Laparoscopic exploration and laparotomy play an important role in the diagnosis of the extent, degree, and severity of tumor burden. Cytology and immunohistochemistry play a key role in the diagnosis of tumor origin and pathological type.
1. Clinical manifestations
The main manifestations include abdominal mass, abdominal distension, ascites, digestive system symptoms, and systemic symptoms.
(1) Abdominal Mass
Abdominal masses in peritoneal metastatic cancer are often multiple and scattered. When metastases are small, abdominal masses are often not palpable. Some tumors are larger, and multiple abdominal masses with varying degrees of mobility can be palpated in different areas during physical examination. Due to the location and pathological nature of the tumor, the mobility, size, and texture vary. Abdominal wall tumors can manifest as fixed masses on the abdominal wall, with a hard texture and significant tenderness.
(2) Abdominal Distension and Ascites
Similar to primary peritoneal tumors, ascites and abdominal distension are the most common clinical symptoms of secondary peritoneal tumors. Abdominal pain appears early, and the amount of ascites is generally small. Physical examination reveals abdominal distension in patients with more ascites, even frog-like abdomen, with positive shifting dullness. Irregular masses can be palpated on palpation. Abdominal puncture and drainage of ascites are colorless or light yellow, slightly turbid, and sometimes bloody ascites, suggesting that tumor tissue may invade blood vessels and cause bleeding or local tissue necrosis and bleeding. PMP is characterized by diffuse "gelatinous ascites" in the abdominal cavity. Cytological examination of ascites reveals tumor cells.
(3) Digestive System Symptoms
Significant digestive system symptoms may be present, with abdominal pain, nausea, and vomiting often being the initial symptoms. Tumor invasion of the abdominal digestive tract and other organs can cause abdominal pain, nausea, vomiting, anorexia, and diarrhea. These symptoms are not obvious in the early stages. As the disease progresses and invades the digestive tract, causing adhesion, obstruction, or even torsion and intussusception, the symptoms become more obvious, manifesting as significant abdominal distension, abdominal pain, nausea, and vomiting. Severe cases may experience shock. When the tumor invades the liver, gallbladder, or pancreas, fever, jaundice, and liver dysfunction may occur.
1649 Peritoneal Tumors Chapter 3 Diagnosis of Peritoneal Tumors
(4) Symptoms of Primary Disease
Mainly secondary to gastric cancer, colorectal cancer, ovarian cancer, and appendiceal mucinous tumors, and may have manifestations of these primary tumors. If the primary disease is gastric cancer, gastrointestinal bleeding, pyloric obstruction, vomiting, and abdominal pain may occur. If it is colorectal cancer, it may manifest as abdominal pain, abdominal distension, vomiting, inability to pass gas, and inability to defecate, indicating intestinal obstruction. If it is ovarian cancer, it manifests as abdominal distension, abdominal pain, menstrual disorders, and irregular vaginal bleeding. When it invades the urinary system, urinary frequency and urgency may occur. Pelvic examination may reveal masses, so pelvic examination and rectal examination should be performed as routine clinical examinations. If it is appendiceal mucinous tumor, it manifests as abdominal distension, abdominal pain, abdominal mass, anorexia, and weight loss.
2. Diagnostic Methods for Secondary Peritoneal Tumors
2.1 Laboratory tests
(1) Tumor Marker Examination
Tumor markers have some auxiliary significance. For primary diseases such as ovarian cancer, gastric cancer, colorectal cancer, and appendiceal mucinous tumors, it is recommended to perform combined detection of multiple markers such as CEA, CA125, and CA199 to provide a reference for clinical diagnosis. For primary gastric cancer, commonly used markers include CEA, CA125, CA199, and CA724. Elevated levels of these markers are positively correlated with peritoneal metastasis, but the sensitivity and specificity for diagnosing peritoneal metastasis are poor, and they are only for clinical reference.
CEA can be used to judge the degree of tumor invasion, CA125 to assess tumor burden and ascites formation, and CA199 to judge the proliferation activity of tumor cells. CEA is highly expressed in gastrointestinal tumors, especially colorectal cancer. Significant elevation suggests metastasis from the gastrointestinal tract. CA125 is mainly used as a marker for ovarian tumors. The ratio of CA125:CEA greater than 25:1 can be used to assess the tumor origin. CA199 is closely related to pancreatic and upper gastrointestinal tumors, but it is also expressed in peritoneal malignant tumors.
(2) Blood Routine and Biochemical Examination
With a large tumor burden and long course of disease, it often manifests as a wasting disease. Blood tests may reveal decreased red blood cells and hemoglobin, and decreased plasma albumin. Routine biochemical tests may reveal various abnormalities, such as abnormal transaminases and bilirubin.
(3) Fecal Occult Blood Screening
When tumors invade the gastrointestinal tract and cause bleeding, fecal occult blood is often positive. The positive rate is higher in peritoneal metastasis secondary to gastrointestinal tumors.
(4) Detection of Tumor Cells in Ascites
For suspected patients, exfoliated cells from ascites or peritoneal lavage fluid cytology can be performed. Ascites cell sediment can also be embedded to make cell wax blocks and paraffin sections. Immunohistochemistry can be used as an auxiliary method if necessary.
Detection of peritoneal metastasis can be clearly diagnosed in those who test positive. Although the sensitivity is relatively low, with a positive rate of 50%~80%, peritoneal puncture has the advantages of simple operation, low cost, high feasibility, and repeatability, and can be used as an effective way to assist in judging the malignancy of tumors. For PMP, microscopy can show a large amount of mucus formation in the ascites, but its high-viscosity gelatinous ascites increases the difficulty of peritoneal puncture and affects the positive rate of the examination.
The following measures can be taken to improve the detection rate of cancer cells in ascites: ① Obtain sufficient ascites/lavage fluid ≥500ml. ② Repeatedly draw ascites or perform peritoneal lavage. ③ When drawing ascites, ask the patient to turn over and change positions, which makes it easier to draw out precipitated cells and thus improves the detection rate of cancer cells.
Cell wax block technology is becoming increasingly prominent in pathology. It involves centrifuging samples of serous cavity effusion, highly concentrating cells and microtissue blocks, and then using fixatives to condense and paraffin-embed them to make sections. In addition to observing the morphology of cancer cells under a light microscope, it is also used for immunocytochemistry and gene detection, etc., which helps in the diagnosis and differential diagnosis of benign and malignant cells, histological types, and the origin of cancer cells, and can improve the sensitivity of pathological diagnosis. For ascites with a high mucus content, this method has a higher positive rate than traditional cytological examination.
2.2 Imaging examinations
(1) Ultrasound
Ultrasound examination has a high detection rate for metastatic tumor ascites and larger metastatic lesions and can be used as an auxiliary tool for the diagnosis of peritoneal metastatic tumors.
The typical ultrasound manifestations of secondary peritoneal tumors are: ① Ascites: fluid-filled dark areas in the abdomen and pelvis. When the amount of ascites is large, ascites can be used as an acoustic window to better observe peritoneal thickening, peritoneal nodules, and other metastatic signs. ② "Omentum cake"-like greater omentum: metastatic tumor lesions of the greater omentum, ultrasound shows that it is significantly thickened and rigid, appearing as a "cake", which is called the "omentum cake" sign. ③ Multiple metastatic lesions: manifested as multiple, unevenly sized hypoechoic nodules on the peritoneum. ④ Primary tumor: primary tumors in organs such as the gastrointestinal tract and ovaries can be found. Ultrasound examination is easily affected by abdominal wall thickness, gastrointestinal gas, gastrointestinal motility, and the examiner's experience. However, the detection rate of peritoneal lesions smaller than 10mm is low, and it is difficult to use it as a qualitative diagnostic basis for peritoneal metastasis.
(2) CT
CT is the preferred diagnostic method, which can observe the size, location, number, nature, and blood supply of metastatic lesions, with a specificity of over 90%.
The main CT manifestations include: ① Ascites: low-density fluid, which can show high density and stratification when combined with bleeding. ② Uneven peritoneal thickening: cord-like thickening or nodules, enhanced scanning shows enhancement. ③ "Omentum cake"-like greater omentum: the greater omentum shows nodular and dirty changes, thickening and enhancement. ④ Single or multiple metastatic lesions: varying in size, shape, and nature; primary tumor signs, see the corresponding chapters of each primary tumor. ⑤ Intestinal invasion: asymmetrical thickening/narrowing and enhancement of the intestine, blurred and increased density of the perintestinal fat space, irregular thickening and enhancement of the mesentery, and possible intestinal obstruction. ⑥ Others: invasion of the urinary system leading to hydronephrosis and ureteral dilatation; invasion of the biliary system, causing intrahepatic and extrahepatic bile duct dilatation; tumor infiltration causing a fan-shaped depression of the liver capsule, which is a characteristic of PMP. The primary lesion of the abdominal organs can be found; for example, in PMP, CT shows omental adhesion masses and mucous ascites, and can also show the primary lesion of the appendix, appendix calcification, or rupture. However, the sensitivity is closely related to the size of the cancer lesion, and the overall sensitivity is not high. When the metastatic lesion is less than 10mm, the sensitivity is 9%~50%; while the sensitivity of nodules less than 5mm is only 11%.
(3) MRI
A meta-analysis showed that MRI combined with DWI can effectively improve the detection rate and diagnostic consistency of small metastatic lesions, with both sensitivity and specificity reaching 90%, and its efficacy is superior to CT.
The main MRI manifestations include: ① Ascites: long T1 and long T2 signals, no enhancement. ② Peritoneal/omental thickening: the parietal layer shows slightly long T1 and equal T2 signals, and various regions including the greater omentum show irregular peritoneal thickening, and obvious enhancement can be seen in T1WI enhanced scanning. ③ Multiple metastatic lesions: nodules/masses vary in size and shape, distributed in different regions, T1WI shows low signal, T2WI shows moderate to high signal, T1WI enhancement shows obvious enhancement, and the boundaries are mostly irregular. ④ Metastatic lesion DWI: metastatic lesions mostly show diffusion restriction, that is, DWI shows obvious high signal, and its derived apparent diffusion coefficient map shows low signal. The shortcomings are that the imaging time is long, it is easily affected by respiratory and motion artifacts, and for patients with poor compliance, MRI examination is limited.
(4) PET/CT
PET/CT can evaluate FDG metabolic changes and improve the detection rate of metastatic lesions. A meta-analysis showed that the sensitivity of PET/CT in diagnosing secondary peritoneal tumors is 87%, and the specificity is 92%. Under PET/CT imaging, secondary peritoneal tumors show high FDG uptake, often multiple lesions, varying in size and irregular boundaries. PMP has less soft tissue component and low FDG uptake, so the diagnostic value of PET/CT is limited.
(5) PET/MRI
PET and MRI provide anatomical, metabolic, and functional information, and have a synergistic effect, which can improve the diagnostic efficacy of peritoneal metastasis. The PCI provided by PET/MRI is closer to the actual PCI than DWI, especially for patients with high tumor burden who have not received chemotherapy. Existing evidence shows that preoperative PET/MRI assessment of peritoneal metastasis can reduce radiation exposure, but requires cooperation between radiology and nuclear medicine departments. Compared with PET/CT and DWI/MRI, the clinical value of PET/MRI needs further research.
(6) Fibroblast activation protein inhibitor PET/CT
Fibroblast activation protein (FAP) is a potential target for the diagnosis and treatment of various malignant tumors. 68Ga or 18F-labeled quinolines (Fibroblast Activation Protein Inhibitor, FAPI) have been developed and validated for the diagnosis of peritoneal metastasis. A meta-analysis showed that 68Ga-FAPI PET may be more accurate than FDG PET/CT in determining PCI, and therefore can be used as a tool to assess the resectability of patients with peritoneal metastasis, but existing studies have a high risk of bias.
Currently, the use of FAPI PET/CT is limited to clinical trials. Large-scale comparative studies and long-term follow-up are needed to determine the clinical value and advantages of FAPI.
(7) Spectral photon counting CT
Spectral Photon Counting CT (SPCCT) achieves direct conversion of single X-ray photons and spectral separation of multiple energy windows through photon counting sensors, providing extremely high spatial resolution and contrast-to-noise ratio, while reducing radiation dose and allowing for multiple contrast imaging. Compared with traditional CT, SPCCT shows higher sensitivity and specificity in the detection of small lesions, and further research is needed.
8. Radiomics and Artificial Intelligence
Radiomics is an image analysis technique that uses computer-aided analysis to analyze physical information that is invisible to the naked eye, such as the grayscale distribution, inter-pixel relationships, and spatial matrix arrangement of images, and uses statistical methods to convert it into quantitative digital features. Based on the correlation between primary tumor CT radiomics features and the risk of peritoneal metastasis, a radiomics model combined with clinical factors has potential application value in assessing the risk of peritoneal metastasis in gastrointestinal cancer, and the prediction efficiency of peritoneal metastasis risk is between 0.7 and 0.9. CT radiomics has the potential to improve the diagnostic level of peritoneal metastasis.
2.3 Pathological Examination
The diagnosis of secondary peritoneal tumors mainly relies on pathological examination, which can clearly identify the histological type of the tumor and is the most direct and accurate means of confirming the pathological type, and has high value for judging the primary tumor.
Pathological biopsy can be divided into image-guided puncture biopsy and laparoscopic biopsy. The former is simple to operate and easy to collect samples, but there may be a risk of dissemination in a few cases. CT or ultrasound-guided puncture biopsy is usually unhelpful for diagnosing PMP, and the puncture may obtain acellular mucus, which may also be the case in other secondary cancers, so percutaneous puncture biopsy is selectively used. If acellular mucus is found, it is highly suggestive of PMP.
Laparoscopy, while performing a biopsy, explores the abdomen and pelvis to determine the size, number, texture, and distribution of metastatic lesions, providing a basis for diagnosis.
Due to the diversity of primary tumors, the pathological types of secondary peritoneal tumors vary, as follows.
1. Peritoneal metastasis of gastric cancer
1) Papillary adenocarcinoma: It has a clear papillary structure, covered with columnar or cuboidal cancer cells, with little to moderate stroma, and cystic dilatation of glands can be seen. It is more common in the early stage of gastric cancer and can evolve into papillary tubular adenocarcinoma (if tubular carcinoma is dominant, it is classified as tubular adenocarcinoma).
2) Tubular adenocarcinoma: According to the degree of glandular duct formation, it is divided into high and moderate differentiation types. High differentiation type: the entire tumor tissue shows a complete and clear glandular duct structure, the tumor cells are columnar, and the stroma is small to moderate. Moderate differentiation type: the glandular duct structure is small or incomplete, occasionally sieve-like structure, the tumor cells are cuboidal or flat, and the amount of stroma varies.
3) Poorly differentiated adenocarcinoma: Glandular duct formation or mucus secretion is only seen in local areas, most cancer cells are arranged in sheets and nests, tumor cells have greater atypia, mitotic figures are easily seen, and necrosis is often seen.
4) Signet ring cell carcinoma: It is called signet ring cell carcinoma if it is mainly or entirely composed of signet ring cells. Cancer cells contain unequal amounts of mucus, the nucleus is eccentric, mostly signet-ring-shaped, and there may be a tendency to form glandular ducts locally. It is the most common in peritoneal metastatic cancer. The histological typing of the mucosal layer and the deep infiltration part is different in some cases, and should be typed according to the dominant principle.
5) Mucinous adenocarcinoma (colloid carcinoma): Contains a large amount of mucus, forming mucus pools in the stroma. Those with more than 50% mucus content can be called mucinous adenocarcinoma, with cancer cells floating in it. Mucinous adenocarcinoma may contain signet ring cell carcinoma components.
6) Special types: including adenosquamous carcinoma, squamous cell carcinoma, hepatoid adenocarcinoma, undifferentiated carcinoma, carcinoma with lymphoid stroma, and carcinoid, etc.
2. Peritoneal metastasis of colorectal cancer can be divided into the following main types.
1) Tubular adenocarcinoma: Papillary invasive growth, showing a glandular duct-like structure, divided into high, medium, and low differentiation according to the proportion of glandular duct formation.
2) Mucinous adenocarcinoma: The proportion of extracellular mucus in the tumor is more than 50%, and there are two main growth patterns: A. Glands are composed of columnar mucus-secreting epithelium, and mucus exists in the inter-glandular lumen; B. Cells are scattered in chains or irregular strings floating in the mucus lake. Mucus can also be seen in the inter-glandular stroma.
3) Signet ring cell carcinoma: Mainly composed of cancer cells containing intracellular mucus, more common in peritoneal metastatic cancer, younger onset, and poor prognosis.
4) Medullary carcinoma: Tumor tissue is arranged in solid sheets and trabeculae, with obvious lymphocytic infiltration. The cytoplasm is abundant and red-stained, and the nucleolus is obvious. It is often accompanied by high microsatellite instability (MSI-H) and belongs to low-grade malignant tumors.
5) Squamous cell carcinoma and adenosquamous carcinoma: Very rare. Adenosquamous carcinoma is composed of adenocarcinoma and squamous cell carcinoma components.
6) Undifferentiated carcinoma: Grows in clumps or diffusely in sheets, without glandular structures or features suggesting glandular differentiation.
7) Other rare types: such as hepatoid adenocarcinoma, serrated adenocarcinoma, micropapillary adenocarcinoma, clear cell carcinoma, etc.
3. Peritoneal metastasis of ovarian cancer
Epithelial cancer is the most common, accounting for 80% to 90%, divided into five subtypes: high-grade serous carcinoma (HGSC) accounts for 70% to 80%, endometrioid carcinoma accounts for 10%, clear cell carcinoma accounts for 10%, low-grade serous carcinoma (LGSC) accounts for 5%, and mucinous carcinoma accounts for 3%.
1) HGSC: The key features are significant cellular atypia and prominent mitotic activity. The nuclei are darkly stained, with obvious atypia, more than three times the original size, and common tumor giant cells. Mitotic figures are easily seen, and the threshold is defined as ≥12 mitotic figures per 10 high-power fields; if there are few mitotic figures, LGSC or other diagnoses should be considered.
2) Endometrioid carcinoma of the ovary: Mostly low-grade, with diverse macroscopic appearances, cystic or solid. Histologically similar to low-grade endometrioid adenocarcinoma of the endometrium. Most have complex glandular, cribriform and (or) papillary structures, exhibiting back-to-back growth, elongated or round glands, with smooth lumens.
3) Clear cell carcinoma: Cystic and solid, mostly unilateral, and large. Nuclei are deeply stained, with obvious atypia, and special hobnail cells can be seen attached to the cyst wall.
4) LGSC: The tumor is indolent, solid or cystic, with fragile papillary excrescences inside or on the surface. LGSC is composed of small papillae covered with cancer cells with relatively uniform nuclei, with a size variation of less than 3 times. Mitotic figures are few, far less than HGSC, with a defined threshold of <12 mitotic figures per 10 HPFs.
5) Mucinous carcinoma: Rare, containing a large amount of mucus, forming mucus pools in the stroma. It often occurs in a single ovary, is more common in young women, is mostly early-stage, and usually does not cause PMP.
6) Rare subtypes of ovarian cancer: Carcinosarcoma and undifferentiated carcinoma, which are highly malignant. The epithelial component is often high-grade serous carcinoma.
(4) PMP
Characterized by the localized or widespread accumulation of viscous gelatinous material in the abdomen and/or pelvis, within the peritoneal cavity. It is mostly a result of the progression of appendiceal mucinous neoplasms. Other primary sites include mucinous neoplasms of the pancreas, urachal remnants of the bladder, and teratomas of the ovary. The diagnostic terminology and histological characteristics of disseminated mucinous neoplasms are detailed in Table 29-3-5. The following is a detailed description: 1) Low-grade (G1, well-differentiated): For stage IV appendiceal mucinous neoplasms, low-grade is synonymous with well-differentiated and G1. Low-grade (G1, well-differentiated) peritoneal tumors are defined as tumors with low-grade cytological morphology and lacking invasive infiltration.
Low-grade (G1, well-differentiated) peritoneal tumors mostly originate from primary low-grade mucinous neoplasms (LAMN).
Disseminated low-grade (G1, well-differentiated) peritoneal tumors are characterized by abundant mucus pools in the peritoneal cavity. The proportion of neoplastic mucinous epithelial components to the total mucinous component of the tumor is <20%. The neoplastic mucinous epithelium mostly appears as strands or small nests with low-grade atypical cytological morphology. Lymph node metastasis is rare; if present, mucinous adenocarcinoma should be considered.
In diagnosing low-grade (G1, well-differentiated) peritoneal tumors, invasive infiltration, signet ring cells, vascular or lymphatic invasion, and peritoneal invasion are not present. If present, mucinous adenocarcinoma should be considered.
Disseminated low-grade (G1, well-differentiated) peritoneal tumors often invade the gastrointestinal wall and may involve the spleen, pancreas, ovaries, omentum, and liver parenchyma. Neoplastic mucinous epithelium and mucus are present in these organs, but this is insufficient for a diagnosis of invasive infiltration, as these tumors typically show "pushing" borders without clear invasive infiltration.
2) High-grade (G2, moderately differentiated): High-grade mucinous adenocarcinoma is defined by the presence of high-grade atypical cytological morphology but lacking signet ring cells. The cellular criteria for high-grade atypia are the same as for other gastrointestinal tracts, including enlarged nuclei, round nuclei, irregular nuclear membranes and chromatin, prominent nucleoli, readily visible mitotic figures, marked (full-thickness) nuclear stratification, loss of nuclear polarity, and glandular complexity (cribriform glands, "back-to-back" glands, and intraluminal papillary clusters).
High-grade (G2, moderately differentiated) mucinous adenocarcinoma demonstrates diffuse high-grade atypia or shows a mixture of low-grade and high-grade atypical areas. The cytological grading within disseminated appendiceal mucinous neoplasms may be heterogeneous, with areas of low-grade atypia mixed with clearly high-grade atypical areas. This heterogeneity suggests that peritoneal tumor lesions require extensive sampling for histological assessment. Invasive, destructive invasion is seen in almost all high-grade (G2, moderately differentiated) mucinous adenocarcinomas.
Histological assessment of destructive invasion within disseminated tumors may be difficult. High-grade (G2, moderately differentiated) mucinous adenocarcinomas often demonstrate high tumor cell density. The latter is defined as the proportion of neoplastic mucinous epithelial components to the total mucinous component of the tumor >20%. Assessment of tumor cell density across the entire section is best performed by reviewing all sections of the case, ideally at low magnification. Low-power assessment of cellular density is often a histological clue to the diagnosis of high-grade (G2, moderately differentiated) mucinous adenocarcinoma. Unlike low-grade (G1, well-differentiated) tumors, approximately 20% of high-grade (G2, moderately differentiated) mucinous adenocarcinomas show lymph node metastasis.
3) High-grade (G3, poorly differentiated) mucinous adenocarcinoma: This tumor usually originates from heterogeneous appendiceal adenocarcinoma, and the most common characteristic feature is the presence of signet ring cell components. Most tumors have >95% signet ring cells; a few cases show a mixture of glandular and signet ring cell morphology.
Invasive, destructive invasion and high tumor density are seen in almost all high-grade (G3, poorly differentiated) mucinous adenocarcinomas.
Unlike high-grade (G2, moderately differentiated) mucinous adenocarcinomas, approximately 70% of high-grade (G3, poorly differentiated) mucinous adenocarcinomas have lymph node metastasis, and most cases have vascular and lymphatic invasion and peritoneal invasion. In rare cases, grade G3 adenocarcinoma presents as solid, sheet-like growth.
Table 29-3-5 Diagnostic Terminology and Histological Characteristics of Disseminated Mucinous Tumors
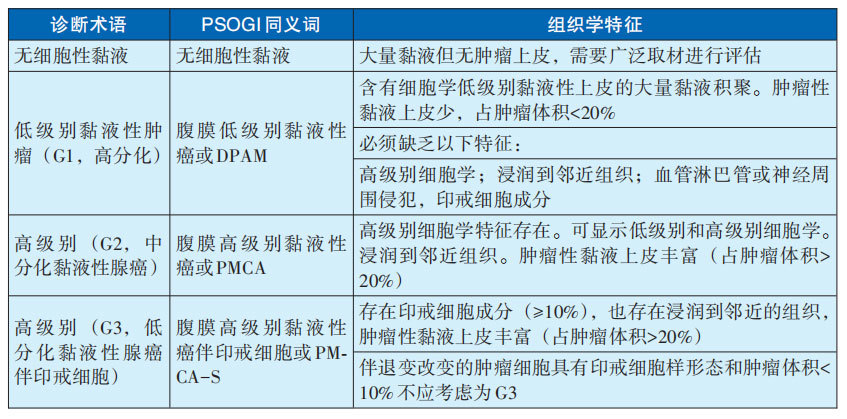
Note: PSOGI: Peritoneal Surface Oncology Group International; DPAM: Disseminated Peritoneal Adenomucinosis; PMCA: Peritoneal Mucinous Carcinomatosis; PMCA-S: Peritoneal Mucinous Carcinomatosis with Signet Ring Cells
2.4 Celiotomy
(1) Laparoscopic exploration
Laparoscopic surgery has become an important tool for diagnosing primary and secondary peritoneal tumors. Laparoscopy makes it relatively easy to find tumor nodules and has a high detection rate for primary tumors invading the serosa or visceral peritoneum, easily obtaining pathological samples for diagnosis. It allows for minimally invasive exploration, lavage to find shed tumor cells, and biopsy for definitive diagnosis. It also assesses the feasibility of optimal CRS under laparoscopy or laparotomy, whether neoadjuvant chemotherapy is needed, avoids unnecessary laparotomy, and guides the selection of laparotomy incision and surgical approach. Laparoscopic examination is minimally invasive, has fewer complications, and allows for faster recovery, making it widely accepted clinically.
Laparoscopic exploration allows for tissue biopsy to determine the origin and pathological type of peritoneal tumors, assess the feasibility of CRS, the extent of CRS, and subsequent treatment. It also allows for the treatment of peritoneal tumors and associated malignant ascites via intraperitoneal hyperthermic perfusion (HIPEC). It also helps understand organ involvement and lymph node metastasis, allowing for surgical treatment while obtaining a pathological diagnosis.
It has important applications in diagnosis, differential diagnosis, and treatment, and is the most direct and accurate method for confirming the pathological type, with high value for the diagnosis and treatment of primary tumors.
Laparoscopy can compensate for the shortcomings of imaging, revealing macroscopic peritoneal metastases and occult intra-abdominal metastases. It allows for direct visualization of tumor location, size, and infiltration range, performing peritoneal tumor index scoring, and assessing the feasibility of CRS. However, there are some limitations: ① There are a few blind spots, making it difficult to observe special areas such as intermesenteric masses and nodules; there is a possibility of false-negative biopsies. ② Lack of tactile sensation, making it impossible to assess the degree of invasion of surrounding organs, and the value of assessing the resectability of the primary lesion is limited.
Attention should be paid to the differentiation of different peritoneal lesions. Peritoneal metastasis of gastric cancer can manifest as scattered, uneven grayish-white nodules, or partially fused patches, commonly found in the diaphragm, mesentery, pelvic wall, etc., and may be accompanied by omental shrinkage and thickening, deep yellow or pale bloody ascites. Peritoneal tuberculosis manifests as diffuse, uniform, raised nodules densely covering the peritoneum, accompanied by surface mucus and grass-green ascites.
Exploration should be conducted strictly in order. If the tumor is located on the posterior wall of the stomach, explore whether it penetrates the serosa and involves adjacent fixed structures. The gastrocolic ligament can be incised with an electrocautery hook to enter the lesser sac to explore whether the transverse mesocolon and pancreatic capsule are invaded. Use long straight forceps to lift the left lateral lobe of the liver to expose the lesser curvature of the stomach and observe whether the tumor penetrates the serosa and involves the lesser omentum. After the examination, the puncture holes should be properly closed, paying attention to no-touch technique to prevent subcutaneous and intramuscular implantation via the puncture pathway.
(2) Laparotomy
Laparotomy is a surgical examination and/or treatment method used by surgeons to find the cause or determine the extent of a lesion and take appropriate surgical action. It is highly invasive and should be chosen cautiously, and can be considered when laparoscopic exploration is difficult. For peritoneal masses that are difficult to diagnose clinically, laparotomy can be used to achieve diagnosis and even treatment.
For some deeper-seated peritoneal tumors, laparoscopic examination may not meet clinical requirements. Laparotomy allows for direct observation of nodules, plaques, and masses on the surface of the peritoneum, greater omentum, mesentery, and abdominal viscera, allowing for surgical treatment while obtaining a pathological diagnosis. Laparotomy has some disadvantages, such as a larger incision and greater trauma, and is less commonly used clinically.
3. Diagnostic Criteria and Differential Diagnosis
3.1 Diagnostic Criteria for Secondary Peritoneal Tumors
For malignant tumors that have undergone surgery or other treatments, the diagnosis of peritoneal metastasis is relatively easy, and it can often be quickly confirmed in conjunction with imaging examinations such as CT. For patients with unexplained abdominal masses or ascites, especially those with multiple abdominal masses, the possibility of secondary peritoneal tumors should be considered. An integrated judgment should be made based on imaging examinations, serum tumor markers, and ascites cytology. Evidence of the primary tumor and pathological biopsy support are the most important basis for confirmation. Highly suspicious cases should undergo laparoscopic examination or laparotomy as early as possible for early treatment.
Main diagnostic criteria: ① History of primary tumor: A clear history of primary tumors in intra-abdominal organs or other sites. ② Symptoms: Ascites, abdominal pain, abdominal masses, anemia, and progressive weight loss. ③ Imaging diagnosis: CT, MRI, PET/CT, etc., provide imaging evidence supporting the diagnosis of secondary peritoneal tumors. ④ Cytological examination of ascites/peritoneal lavage fluid: Detection of tumor cells. ⑤ Puncture biopsy, laparoscopic or open surgical direct visualization biopsy: Supports the diagnosis of secondary peritoneal tumors.
3.2 Differential Diagnosis of Secondary Peritoneal Tumors
Secondary peritoneal tumors often have a history of primary tumors, or imaging evidence of primary tumors is found at initial diagnosis. CT findings are similar to malignant pleural mesothelioma (MPM), but MPM often has a history of asbestos exposure, and tissue calcification is more obvious than in peritoneal metastatic cancer, and lymph node metastasis is rare. Peritoneal metastatic cancer should also be differentiated from primary peritoneal cancer. Clinically, primary peritoneal serous adenocarcinoma is often misdiagnosed as metastatic ovarian serous adenocarcinoma, and should be ruled out based on medical history and pathological biopsy results.
1657 Peritoneal Tumor Chapter 3 Diagnosis of Peritoneal Tumors
(1) Tuberculous peritonitis
Tuberculous peritonitis and secondary peritoneal tumors have the following main differences: ① Tuberculosis may have prolonged low-grade fever, with positive tuberculin bacillus detection in ascites and positive tuberculin test. ② Tuberculous peritonitis leads to enlarged lymph nodes, where calcification or necrosis foci are easily found, and CT examinations should be used to distinguish them. ③ The density of ascites produced by tuberculosis is higher, and the CT value is mostly between 20-45 HU. ④ In addition, miliary microabscesses in the liver and spleen can be found in tuberculous peritonitis.
(2) Hepatocellular Carcinoma Ascites
Some patients are diagnosed with hepatocellular carcinoma due to ascites, and it is not difficult to differentiate based on the patient's physical signs and imaging examinations.
(3) Inflammatory Pseudotumor of the Peritoneum
It is mainly composed of fibrous components, is rare clinically, and is characterized by low signal intensity on T1WI and T2WI MRI.
(4) Primary Sclerosing Peritonitis
Rare, mainly occurring in patients on long-term peritoneal dialysis; diagnosis is not difficult based on medical history and relevant imaging examinations.
Section 3 Staging Standards and Scoring Scales for Primary Peritoneal Tumors (Staging of secondary peritoneal tumors refers to the staging of primary peritoneal tumors)
1 AJCC 8th Edition Staging (Applicable to ovarian and fallopian tube tumors and primary peritoneal carcinomas, see Table 29-3-6)
Table 29-3-6 AJCC Staging
2. Pathological Staging of Peritoneal Tumors (See Table 29-3-7)
Table 29-3-7 Pathological Staging of Peritoneal Tumors
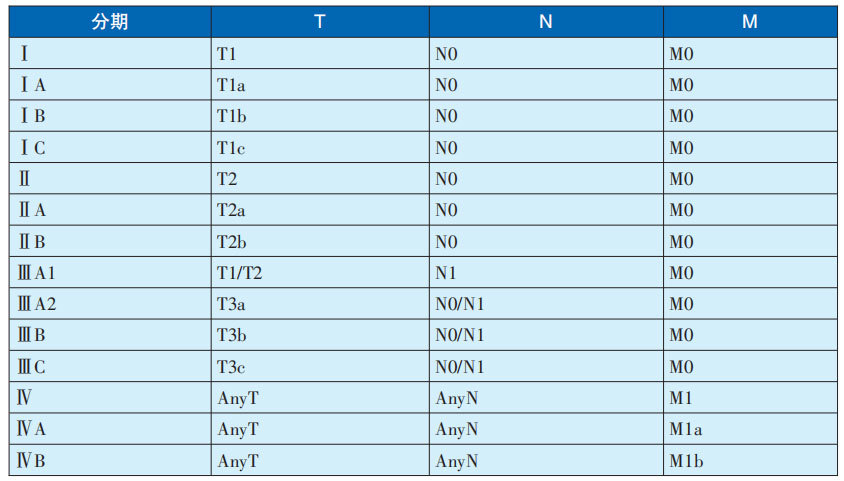
3. Peritoneal Cancer Index (PCI) Staging
The Peritoneal Cancer Index (PCI) is a commonly used clinical staging system for peritoneal tumors. This method divides the abdomen into 13 regions: using a horizontal line through the lowest points of the bilateral costal arches, a horizontal line through the highest points of the bilateral anterior superior iliac spines, and the bilateral mid-clavicular lines to divide the abdominal cavity into 9 regions (0~8), namely: left and right upper abdomen, upper abdomen, left and right lumbar regions, central region, left and right iliac fossae, and pelvic floor; the small intestine is divided into 4 regions (9~12), namely: upper and lower jejunum, upper and lower ileum. A total of 13 regions are divided, and each region's lesion size (LS) is scored. The sum of the LS scores in each region is the PCI score, with a total score of 0-39 points.
Tumor LS score within the region:
(1) No visible tumor, score 0.
(2) Tumor diameter <0.5cm, score 1.
(3) Tumor diameter 0.5~5.0cm, score 2.
(4) Tumor diameter >5.0cm or tumor fusion, score 3.
Surgery should be considered cautiously when PCI >20. The PCI index is closely related to long-term survival rate, and it is not only important for predicting survival rate, complication rate and mortality rate, but also closely related to the efficacy of CRS, HIPEC and other treatments. Although it is not feasible to detect the number of diffuse peritoneal metastases, the PCI index is still a relatively reasonable method for evaluating the severity of peritoneal tumors.
Chapter 4 Treatment of Peritoneal Tumors
The treatment of peritoneal tumors varies depending on the origin of the tumor, but integrated surgical treatment mainly based on CRS combined with HIPEC can significantly improve prognosis and achieve satisfactory results.
Chemotherapy is the most commonly used adjuvant therapy. Targeted therapy, immunotherapy, radiotherapy, traditional Chinese medicine, and nutritional support can also be selectively used according to the patient's condition.
The main treatment recommended in this guideline is CRS+HIPEC; other treatments will be introduced separately.
Table 29-4-1 Treatment Methods for Peritoneal Tumors
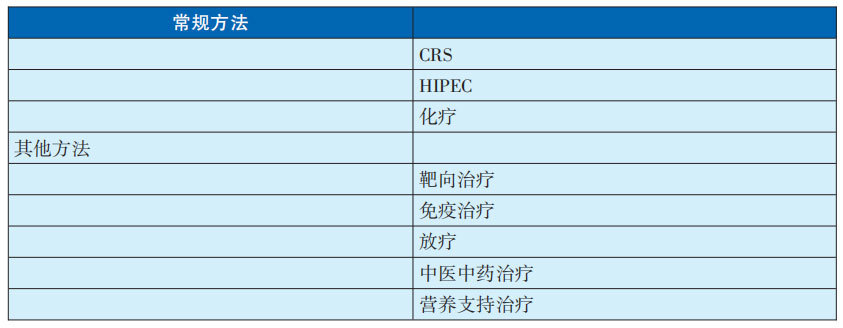
Section 1 CRS combined with HIPEC
CRS combined with HIPEC uses surgery, hyperthermia, local chemotherapy, and peritoneal lavage to establish a new integrated treatment strategy for peritoneal tumors. CRS can remove macroscopically visible tumors in the abdominal cavity, and HIPEC has a clearing and killing effect on residual microscopic cancer foci after surgery, and has a unique effect on peritoneal tumors and malignant ascites caused by them.
1. CRS
(1) Definition of CRS
CRS refers to the surgical removal of all macroscopically visible tumors in the abdominal cavity as completely as possible to reduce the tumor burden. That is, removing all tumors from the parietal and visceral peritoneum, including affected organs or tissues and peritoneum, as well as related regional lymph node dissection, with the goal of reducing the largest diameter of residual tumors to less than 0.25cm.
Integrating perioperative treatment, overall patient condition, extent of peritoneal spread, distant metastasis of lesions, surgical risk and complications, not all lesions can be removed. Before undergoing CRS, patients should undergo a comprehensive assessment and record PCI.
(2) CRS Methods
CRS refers to the complete resection of organs, tissues, and peritoneum involved by the tumor. The recommended order of tumor resection for CRS is: hepatic round ligament, greater omentum, lesser omentum, right upper abdomen, left upper abdomen, diaphragmatic peritoneum, lateral wall peritoneum, right iliac fossa, left iliac fossa, pelvic floor peritoneum, and small bowel mesentery.
The operations required for maximum CRS include: ① Regional en bloc resection of the parietal peritoneum. ② Resection of the visceral peritoneum and affected organs. ③ The gallbladder fossa, splenic fossa, and Douglas' pouch are prone to tumor implantation. Combined with the patient's overall condition, the diseased gallbladder, spleen, rectum, and uterine appendages should be resected.
If the visceral peritoneum is invaded, it is necessary to combine the resection of part of the stomach, small intestine, or colorectum. If the pylorus is fixed to the retroperitoneum at the pylorus, tumor cells often accumulate in the subpyloric space through the epiploic foramen, leading to gastric outlet obstruction. When the lesser omentum and subpyloric space tumors fuse, total gastrectomy is required to achieve satisfactory CRS. The ileocecal region has a small range of activity, and when tumor cells invade, the terminal ileum and right hemicolon need to be resected. When the pelvis is invaded, tumor cells often invade the sigmoid colon and rectum, and pelvic peritonectomy requires stripping of the pelvic side wall peritoneum, bladder surface peritoneum, and resection of part of the sigmoid colon and rectum.
(3) CRS Evaluation Criteria
After CRS surgery, the completeness of cytoreduction (CCR) is evaluated, generally using the CCR scoring method.
The specific scoring rules are: ① CCR-0 points: no visible tumor nodules after surgery. ② CCR-1 point: residual tumor diameter <0.25cm. ③ CCR-2 points: residual tumor diameter 0.25~2.5cm. ④ CCR-3 points: residual tumor diameter >2.5cm or unresectable lesions in any part of the abdomen.
Residual tumor diameter less than 0.25cm (CCR-0 and CCR-1) is considered satisfactory CRS.
2. HIPEC
(1) HIPEC Definition
HIPEC is a treatment technique that involves heating a chemotherapy drug-containing perfusion solution to a therapeutic temperature, perfusing it into the patient's abdominal cavity and maintaining it for a certain period of time to prevent and treat peritoneal tumors and the resulting malignant ascites. It has been used in the treatment of secondary and primary peritoneal tumors such as gastric cancer, colorectal cancer, ovarian cancer, hepatobiliary pancreatic cancer, PMP and MPM.
(2) HIPEC Principle
1) Cancer cells in a 43℃ environment, continuously soaked and flushed with liquid, can experience irreversible thermal damage. Normal tissues can tolerate 47℃ high temperature for 1 hour. The difference in temperature tolerance between different tissues is used to perform targeted tumor killing at a specific temperature.
2) The multiple thermal effects of HIPEC can lead to thrombosis of tumor blood vessels, inhibit tumor angiogenesis and destroy tumor cell homeostasis, causing tumor cell degeneration and necrosis.
3) Thermal therapy can enhance the cytotoxicity of chemotherapeutic drugs to tumor cells, enhancing drug sensitivity and penetration.
4) Continuous peritoneal lavage can physically flush free cancer cells and peritoneal micrometastases in the peritoneal cavity, removing residual cancer cells and free lesions in the peritoneal cavity.
5) Heat shock proteins can be further activated under the effect of warmth, inducing the body's tumor-controlling immune response, leading to tumor protein denaturation.
(3) HIPEC Technical Methods
Open HIPEC and closed HIPEC.
Open refers to placing a thermal perfusion tube, 2 outflow tubes and 2 inflow tubes, a total of 4 tubes, after the completion of laparotomy or exploration. Under open conditions, continuous peritoneal lavage and perfusion are performed. During the process, the peritoneal perfusion fluid can be dynamically stirred under manual operation to ensure uniform perfusion fluid temperature and sufficient soaking of the interspaces in the peritoneal cavity.
Closed is used after laparoscopic surgery or exploration. Under laparoscopic or open visualization, 4 perfusion tubes are placed, 2 in and 2 out, and continuous peritoneal lavage and perfusion are performed in the closed peritoneal cavity.
(4) HIPEC Technical Standard Parameters and Operating Procedures
1) Open or closed: Open or closed abdomen.
2) Chemotherapy drugs: Drugs sensitive to the primary tumor, with high permeability, high molecular weight, low peritoneal absorption rate, synergistic effect with thermal effect, and low peritoneal irritation.
3) Chemotherapy drug dosage: Refer to systemic chemotherapy dosage.
4) Temperature: 43±0.1℃.
5) Time and frequency: 60~90min, the interval between each treatment is not less than 24 hours; prophylactic: 1~2 times, therapeutic: 1~3 times, 3~5 times can be considered as appropriate.
6) Capacity: 2L/m², the effective perfusion fluid is generally 4~6 L, based on peritoneal filling.
7) Speed: 400~600ml/min.
(5) HIPEC Indications and Contraindications
Indications: ① Age 20~75 years old, those over 75 years old but in good general condition can also be considered. ② KPS score >70. ③ Intraoperative detection of free cancer cells positive. ④ Peritoneal metastasis (PCI<20). ⑤ High-risk peritoneal dissemination patients, such as T3, T4 stage tumors, lymph node metastasis, adenocarcinoma with signet ring cell carcinoma, tumor perforation/rupture, invasion of adjacent organs or accompanied by vascular/lymphatic vessel emboli, nerve invasion, etc.
Contraindications: ① Age >75 or <20 years old, relative contraindications. ② Anastomosis with edema, ischemia, tension and other poor healing factors. ③ Extensive adhesions in the abdominal cavity caused by various reasons. ④ Multiple metastases in distant organs (liver, lung, brain or bone, etc.) found in preoperative routine examinations. ⑤ Moderate or severe stenosis of the mesentery or with obvious contraindications for routine surgery.
(6) HIPEC Drug and Perfusion Fluid Selection
Intraperitoneal administration has better pharmacokinetic activity than intravenous administration. Drugs must have direct cytotoxic activity, synergistic effect with thermal therapy and low systemic toxicity. According to the characteristics of chemotherapeutic drugs, patient conditions, and tumor sensitivity, appropriate drugs are selected for HIPEC treatment (specific drugs are detailed in the subsequent description of each tumor). The drug dosage is generally based on systemic chemotherapy, and can be adjusted appropriately according to age, physical condition, tolerance and bone marrow proliferation ability. Hydration treatment is routinely performed with cisplatin, and sodium thiosulfate can alleviate cisplatin nephrotoxicity. Routine allergy prevention is performed with paclitaxel. The perfusion fluid generally selects physiological saline, 5% glucose, distilled water, etc. The total volume of liquid is 2L/m², and the effective perfusion fluid is controlled at 4~6L to maintain sufficient peritoneal perfusion and build a complete circulatory system. Due to their special nature, carboplatin and oxaliplatin are easily diluted with physiological saline, which can lead to unstable drug efficacy. Therefore, 5% glucose is used as the diluent, but for patients with diabetes, it should be used cautiously or not.
(7) HIPEC Treatment Mode
The clinical application of HIPEC is becoming increasingly refined and standardized. Domestic scholars have developed China Hyperthermic Intraperitoneal Chemotherapy (C-HIPEC) technology with the advantages of high precision, large capacity, constant temperature perfusion, and continuous circulation, and proposed the C-HIPEC model for tumor treatment, including preventive mode, therapeutic mode and conversion mode:
1) Preventive mode, curative intent surgery (CIS) CIS+HIPEC, i.e., C-HIPEC, is suitable for high-risk populations with peritoneal metastasis after receiving CIS. HIPEC treatment can prophylactically clear micrometastases, subclinical lesions and free cancer cells in the peritoneal cavity, prevent the occurrence of peritoneal tumors, and improve the cure rate.
2) Therapeutic mode, CRS+HIPEC, i.e., C-HIPEC, is suitable for peritoneal tumor patients after CRS surgery. After HIPEC treatment, those with satisfactory cell killing (CCR-0, CCR-1) strive to achieve clinical cure, while those with unsatisfactory results (CCR-2, CCR-3) prolong survival and improve quality of life.
3) Conversion mode, Conversion + HIPEC, namely C-HIPEC, is suitable for patients with a large amount of ascites or extensive peritoneal metastasis at the first diagnosis. After HIPEC combined with systemic treatment, the tumor lesions are reduced and shrunk, striving to convert to CRS + HIPEC.
Section 2 Treatment of Primary Peritoneal Tumors
The treatment of primary peritoneal tumors includes the treatment of primary peritoneal cancer and MPM.
1. Treatment of Primary Peritoneal Cancer
Integrated treatment based on CRS + HIPEC is recommended for primary peritoneal cancer. Postoperative chemotherapy regimens are selected based on pathological diagnosis, staging, and grading. After a comprehensive assessment of the patient's condition, patients who can achieve satisfactory CRS can undergo CRS + HIPEC first, followed by adjuvant chemotherapy; if satisfactory CRS cannot be achieved, neoadjuvant chemotherapy (2-3 cycles) can be performed first. When the tumor shrinks to meet the surgical requirements, CRS + HIPEC is performed promptly, followed by continued adjuvant chemotherapy (a total of 6-8 cycles).
1.1 CRS + HIPEC
The surgical procedure for primary peritoneal cancer is mainly CRS, striving to completely remove all macroscopically identifiable tumors on the parietal and visceral peritoneum to achieve satisfactory CRS. If complete resection is not possible, the residual tumor diameter should be controlled to within 1 cm as much as possible.
After CRS surgery, HIPEC treatment is recommended in the absence of obvious contraindications. Commonly recommended chemotherapeutic drugs for HIPEC include: oxaliplatin, mitomycin, cisplatin, docetaxel, gemcitabine, irinotecan, etc.
1.2 Chemotherapy
The biological behavior has similar histological and clinical characteristics and dissemination patterns to advanced ovarian cancer, and the treatment principles refer to the chemotherapy regimens for ovarian cancer. According to the 2024 NCCN guidelines for ovarian cancer, the first-line chemotherapy for ovarian cancer is the TC regimen (paclitaxel 175 mg/m² + carboplatin AUC 5-6 ± bevacizumab 7.5 mg/m² or 15 mg/m², at least 6 courses). Patients achieving CR/PR with chemotherapy receive bevacizumab maintenance therapy, which is also recommended for patients with primary peritoneal cancer. Patients with high surgical risk can consider neoadjuvant therapy first, using intravenous TC regimen chemotherapy.
1.3 Targeted Therapy
For patients sensitive to platinum-based drugs, bevacizumab combined with platinum-based chemotherapy can also be the first choice for these patients.
For recurrent patients who achieve CR/PR with platinum-based chemotherapy + bevacizumab, bevacizumab can be continued as maintenance therapy. Some patients have BRCA mutations or homologous recombination deficiency (HRD), and poly ADP ribose polymerase (PARP) inhibitors can be selected for maintenance therapy.
1.4 Radiotherapy
Radiotherapy is rarely used in primary peritoneal cancer. When patients have contraindications to surgery and chemotherapy, or when local symptoms are more obvious, it can be considered, mostly palliative radiotherapy to relieve pain and symptoms. Methods include external irradiation and radioactive particle implantation. The decision on the regimen and dose selection varies from person to person, and it is recommended to make decisions after discussion by a multidisciplinary team (MDT to HIM).
Whole abdominal radiation therapy (WART) of 30 Gy/15 F may be helpful for patients after CRS, but the radiation tolerance of the liver, kidneys, and intestines severely limits the radiation dose. WART shows significant efficacy only in a small number of patients, especially early non-serous peritoneal cancer with CCR-0.
2. Treatment of MPM
Integrated treatment based on CRS + HIPEC is recommended for MPM. CRS removes as much visible tumor lesions in the abdominal cavity as possible, and HIPEC can remove residual free cancer cells, micrometastases, and subclinical lesions after surgery. Chemotherapy, radiotherapy, and targeted therapy play an auxiliary role in the integrated treatment of MPM.
2.1 CRS + HIPECa
CRS should be performed as early as possible for MPM to completely remove the tumor. When the tumor is large and widely disseminated, the main tumor should be removed as much as possible to reduce the tumor burden. When the disease progresses to cause intestinal obstruction and the main tumor cannot be removed, intestinal stoma surgery should be considered to relieve the intestinal obstruction. If there are no surgical contraindications for recurrence, active surgical treatment can still be performed.
CRS combined with HIPEC is significantly effective in MPM patients. The chemotherapeutic drugs used for HIPEC in MPM include: cisplatin, pemetrexed, etc.
Note a:
① A 2009 J Clin Oncol report on a clinical study of 405 MPM patients from 8 international multicenters (median PCI score: 20 points), 372 patients received CRS + HIPEC, with a median survival time of 56 months.
② At the 9th International Conference on Peritoneal Surface Tumors held in Amsterdam, Netherlands in 2014, the Peritoneal Surface Oncology Group International (PSOGI) officially proposed the CRS + HIPEC strategy as the standard treatment for MPM.
③ In 2015, the authoritative international medical journal CA Cancer J Clin summarized the latest progress in treatment. For MPM patients who received satisfactory CRS surgery combined with HIPEC, the average survival time was 38 to 90 months or more, while only 12 months for those receiving systemic chemotherapy.
2.2 Chemotherapy
For MPM that cannot be surgically treated, refer to the 2024 NCCN guidelines for peritoneal mesothelioma. First-line regimens can include: pemetrexed + cisplatin, pemetrexed + cisplatin + bevacizumab. Carboplatin can be used as a substitute for cisplatin in patients intolerant to cisplatin. In specific cases, first-line chemotherapy regimens can also include: gemcitabine + cisplatin, pemetrexed monotherapy, vinorelbine monotherapy. Although gemcitabine combined with cisplatin is effective for MPM, the incidence of grade 3-4 neutropenia is 60%, so careful selection is recommended.
Second-line regimens can include immunotherapy (single or double immunotherapy). If pemetrexed is effective in the first line, pemetrexed can still be selected for cross-line chemotherapy in the second line. Gemcitabine or vinorelbine monotherapy can also be used as alternative second-line chemotherapeutic drugs.
Whether neoadjuvant and adjuvant chemotherapy for CRS + HIPEC benefit patients still needs to be verified.
2.3 Targeted Therapy
Targeted therapy has not made a breakthrough. For patients with EGFR gene overexpression, ALK, BAP1, NF2, and ALK gene mutations, related drugs such as nintedanib, EZH2 inhibitors, and ALK inhibitors have shown good clinical prospects in phase I/II clinical trials, but there are no breakthrough phase III clinical trial results yet.
2.4 Immunotherapy
Referring to the clinical research results of pleural mesothelioma and the 2024 NCCN guidelines for peritoneal mesothelioma, nivolumab + ipilimumab is the preferred immunotherapy regimen for biphasic or sarcomatoid mesothelioma. If MPM patients choose chemotherapy as the first-line regimen, nivolumab ± ipilimumab immunotherapy can be selected as the second-line treatment.
A phase II clinical study in 2021 showed that atezolizumab + bevacizumab as a second-line treatment for MPM had an ORR of 40% and a 1-year OS of 85%. Currently, this regimen is only suitable for patients who have not received immunotherapy in the frontline.
2.5 Radiotherapy
The value of radiotherapy is currently uncertain. Some studies have confirmed that whole-abdominal radiotherapy after surgery or chemotherapy can improve median OS and quality of life, but the efficacy is limited, and the poor tolerance of important abdominal organs and numerous adverse reactions (intestinal adhesions, intestinal obstruction) are the main reasons hindering the application of radiotherapy in MPM. It is recommended that the use of radiotherapy should be discussed by MDT to HIM to determine the appropriate radiotherapy techniques and doses.
Section 3 Treatment of Secondary Peritoneal Tumors
1 Treatment of Gastric Cancer Peritoneal Metastasis
Peritoneal metastasis of gastric cancer is often secondary to advanced gastric cancer, formed by direct implantation from the primary lesion through the serosa or by lymphatic or hematogenous dissemination. The condition is complex, involving multiple organs and affecting multiple systems. The prognosis is poor, and it is the leading cause of death in advanced gastric cancer. The more severe the metastasis, the worse the prognosis. The natural course is extremely short, with a median OS generally not exceeding 1 year, and only 3.3 months for those with other metastases. The treatment goals are mainly to alleviate pain, improve quality of life, and prolong survival. Integrated treatment is mainly selected, including CRS+HIPEC, systemic chemotherapy, intraperitoneal chemotherapy, molecular targeted therapy, and immunotherapy.
1.1 CRS + HIPEC
Satisfactory CRS is often limited to gastric cancer peritoneal metastasis with smaller areas of early invasion or more localized metastatic lesions. Improving the early detection rate is extremely important for obtaining satisfactory surgical efficacy. However, many patients are found to have diffuse peritoneal metastasis, which is difficult to achieve satisfactory surgical resection, especially when combined with other organ metastases. Palliative surgery is commonly used to reduce tumor burden, relieve symptoms, reduce the risk of complications such as bleeding and perforation from the primary lesion, and create opportunities for integrated treatment.
HIPEC treatment for gastric cancer peritoneal metastasis often uses oxaliplatin, mitomycin, cisplatin, docetaxel, and irinotecan.
(1) Prevention mode: CIS+HIPEC
Gastric cancer with high-risk factors for peritoneal metastasis, after radical resection, undergoes 1-2 HIPEC treatments to remove intraoperative free cancer cells and subclinical lesions. Many clinical studies have shown that this can improve survival rate, but further phase III studies are needed to confirm this.
(2) Treatment mode: CRS+HIPECb
Suitable for patients with relatively localized peritoneal metastasis, low PCI score (<20 points), and good tolerance. CRS combined with HIPEC, without increasing surgical complications and mortality, especially for those with relatively localized peritoneal metastasis and satisfactory CRS, can significantly improve survival rate after HIPEC treatment.
(3) Conversion mode: Conversion+HIPECc
Suitable for patients with extensive peritoneal metastasis or a large amount of ascites at the first diagnosis. HIPEC, as a conversion therapy, can remove or reduce metastatic cancer nodules, and combined with systemic therapy can reduce and shrink peritoneal metastasis and primary lesions, converting to CRS+HIPEC, improving quality of life and survival rate. Further phase III studies are needed to confirm this.
Note b:
①Prospective clinical research results show that compared with 6.5 months in the CRS group, the median survival time in the CRS+HIPEC treatment group was significantly prolonged to 11.0 months.
②In 2019, J Clin Oncol reported a clinical study of gastric cancer peritoneal metastasis. The median survival time in the CRS+HIPEC group was 18.8 months, and the 5-year OS reached 19.9%, significantly better than the control group's 12.1 months and 5-year OS of 6.4%.
③2024 NCCN Guidelines for Gastric Cancer: HIPEC may be effective for carefully selected patients with low-volume peritoneal metastasis, and further clinical research is needed.
Note c:
①In 2019, a report on 71 cases of gastric cancer peritoneal metastasis undergoing laparoscopic HIPEC treatment: Laparoscopic intraperitoneal hyperthermic chemotherapy (LS-HIPEC) is a new strategy for treating gastric cancer peritoneal metastasis, is safe for patients, and may help perform gastrectomy.
②In 2020, multicenter clinical data in China showed that HIPEC can increase the median survival time of gastric cancer peritoneal metastasis from 10.8 months to 15.9 months, and the 3-year OS increased by 8.3%, which is expected to improve the conversion success rate of patients.
③Several domestic expert consensuses recommend HIPEC treatment for gastric cancer peritoneal metastasis.
1.2 Chemotherapy
Systemic chemotherapy is an effective treatment for advanced gastric cancer, which can control disease progression, relieve symptoms, reduce staging, increase surgical resection rate, and improve overall efficacy. Fluorouracil is used as the basis, combined with platinum and/or taxanes to form two-drug/three-drug regimens.
(1) First-line treatment regimen
①XELOX (3 weeks/cycle): Oxaliplatin 130mg/m² intravenous infusion d1; Capecitabine 1000mg/m² bid oral d1~14.
②FOLFOX (2 weeks/cycle): Oxaliplatin 85mg/m² intravenous infusion d1; Calcium folinate 400mg/m² intravenous infusion d1; 5-Fu 400mg/m² intravenous infusion d1, followed by 2400~3600 mg/(m²·d) civ 46h.
③SOX (3 weeks/cycle): Oxaliplatin 130mg/m² intravenous drip d1; tegafur 40mg/m² bid oral d1~14.
④DF (4 weeks/cycle): Cisplatin 75~100mg/m² intravenous drip d1; fluorouracil 75~1000mg/m² continuous infusion d1~4.
⑤DCF regimen (2 weeks/cycle):
A. Docetaxel 40mg/m² intravenous drip d1; calcium folinate 400mg/m² intravenous drip d1; 5-Fu 400mg/m² intravenous drip d1, followed by 1000 mg/(m²·d) civ d1-d2; cisplatin 40mg/m² intravenous drip d3.
B. Docetaxel 50mg/m² intravenous drip d1; oxaliplatin 85mg/m² intravenous drip d1; 5-Fu 1200 mg/(m²·d) civ d1-d2.
Note: For other chemotherapy regimens, please refer to the "Principles of Systemic Treatment" in the 2024 NCCN Gastric Cancer Guidelines.
(2) Second-line treatment regimens
①Paclitaxel/Docetaxel monotherapy
A. Docetaxel 75~100mg/m² intravenous drip d1, 3-week cycle; 1668 Chinese Oncology Integrated Diagnosis and Treatment Guidelines B. Paclitaxel 135~250mg/m² intravenous drip d1, 3 weeks/cycle; or Paclitaxel 80mg/m² intravenous drip d1, once weekly, 4-week cycle; or Paclitaxel 80mg/m² intravenous drip d1, d8, d15, 4 weeks/cycle.
②Irinotecan monotherapy
Irinotecan 250~350mg/m² intravenous drip d1, 3 weeks/cycle; or Irinotecan 150~180mg/m² intravenous drip d1, 2 weeks/cycle; or Irinotecan 125mg/m² intravenous drip d1, d8, 3-week cycle.
Note: For other chemotherapy regimens, please refer to the "Principles of Systemic Treatment" in the 2024 NCCN Gastric Cancer Guidelines.
1.3 Intraperitoneal chemotherapy
Chemotherapy drugs are directly administered into the peritoneal cavity to act on tumor cells, without passing through the blood-peritoneal barrier, allowing for full contact with the lesions. The PHOENIX study is the first phase III clinical study on intraperitoneal chemotherapy for peritoneal metastasis of gastric cancer, suggesting that patients with moderate or more ascites can benefit significantly from survival, providing a new treatment idea for patients, namely neoadjuvant intraperitoneal and systemic chemotherapy (NIPS). Many domestic studies have further confirmed its safety and effectiveness. NIPS has certain application prospects, but large-sample prospective randomized controlled clinical studies need to be carried out.
1.4 Targeted therapy
(1) First-line treatment regimen
Mainly used as a supplementary treatment. Trastuzumab targets HER-2, inducing tumor cell death and inhibiting tumor cell proliferation. According to the 2024 NCCN Gastric Cancer Treatment Guidelines, for patients with PD-L1 CPS ≥1, pembrolizumab + trastuzumab + XELOX or DF regimen is recommended; for patients with PD-L1 CPS <1, trastuzumab + XELOX or DF regimen is recommended.
(2) Second-line treatment regimens
Ramucirumab (anti-VEGFR2 monoclonal antibody) monotherapy or in combination with paclitaxel is recommended by the 2024 NCCN Gastric Cancer Guidelines (Class 1 evidence) as a second-line treatment regimen, with specific dosages as follows: Ramucirumab 8mg/kg, intravenous drip, d1 and d15 + paclitaxel 80mg/m², intravenous drip, d1, 8, 15, every 4 weeks as one cycle; Ramucirumab 8mg/kg, monotherapy, intravenous drip, d1, every 2 weeks as one cycle. In addition, based on the results of the DESTINY-Gastric 01 and 02 studies, the HER2-targeted antibody-drug conjugate (ADC) tucatinib monotherapy has been recommended by the 2024 NCCN Gastric Cancer Treatment Guidelines for second-line and subsequent treatments.
(3) Third-line treatment regimens
Apatinib mesylate (VEGFR-2 small molecule tyrosine kinase inhibitor) is recommended as a third-line or later treatment regimen for advanced gastric cancer or adenocarcinoma of the gastroesophageal junction, regardless of HER2 expression.
Based on the results of the C008 study of the domestically produced ADC drug vicditinib and the DES-TINY-Gastric 06 study of tucatinib conducted in China, both vicditinib and tucatinib are currently used as third-line and subsequent treatment regimens for HER2-positive advanced gastric cancer.
In addition, the 2024 NCCN Gastric Cancer Treatment Guidelines recommend the use of entrectinib or larotrectinib, dabrafenib and trametinib, and ceptinib for the treatment of patients with NTRK gene fusion, BRAF V600E mutation, and RET gene fusion advanced gastric cancer, respectively.
1.5 Immunotherapy
In the past 3 years, many phase III RCT studies at home and abroad have confirmed that the first-line treatment for HER2-negative advanced gastric cancer is immunotherapy combined with XELOX or FP regimen chemotherapy, in which immunotherapeutic drugs can use nivolumab, pembrolizumab, sintilimab, tiragolumab or sugemalimab, regardless of the expression status of PD-L1.
For patients with MSI-H and dMMR gastric cancer peritoneal metastasis, pembrolizumab, nivolumab ± ipilimumab can be used for first-line, second-line or third-line treatment. Domestic immunotherapeutic drugs such as envolimab, tiragolumab, and serulimab are only recommended for second-line treatment, regardless of HER2 expression status.
1.6 Radiotherapy
Gastric cancer peritoneal metastasis is generally multifocal, clonally distributed in multiple areas of the peritoneal cavity or even throughout the entire peritoneal cavity. Radiotherapy alone often does not achieve satisfactory results. Radiotherapy is often used as a palliative treatment to relieve symptoms, improve local control and improve quality of life.
If radiotherapy is considered, the regimen needs to be determined after discussion by MDT to HIM. Postoperative palliative resection of gastric cancer followed by radiotherapy alone can effectively improve the local control rate. It is of higher value for patients with a higher risk of local recurrence. The recommended irradiation dose for gastric cancer radiotherapy is 45~50.4 Gy/25~28fx. Under the premise of properly protecting adjacent intestinal tracts and other organs at risk, the dose can be increased to 54~60 Gy for local or residual lesions after chemotherapy. For more detailed radiotherapy techniques and doses, please refer to the relevant sections of the gastric cancer guidelines.
2 Treatment of colorectal cancer peritoneal metastasis
2.1 CRS+HIPEC
Peritoneal metastasis of colorectal cancer has a poor overall prognosis and is primarily treated with systemic therapy. For patients with a smaller tumor burden, in addition to systemic therapy, integrated treatment with CRS+HIPEC can be considered, which can significantly prolong PFS and OS and has become a standard treatment method.
(1) Prevention mode: CIS+HIPEC
For patients with colorectal cancer with high-risk factors for peritoneal metastasis, after radical surgery, preventive HIPEC treatment 1-2 times can remove free cancer cells and subclinical lesions during surgery. Many clinical studies have shown that this can improve survival rates, but further phase III studies are needed to confirm this.
(2) Treatment Mode: CRS+HIPEC
For peritoneal metastasis of colorectal cancer, CRS should be performed to the greatest extent possible. Peritoneal metastatic lesions and tumor-involved organ tissues need to be resected. When combined organ resection is required, gastrectomy, partial small bowel resection, colorectal resection, partial pancreatectomy, partial hepatectomy, cholecystectomy, splenectomy, nephrectomy, ureterectomy, cystectomy, hysterectomy, oophorectomy, etc., should be performed as needed.
Chemotherapy drugs used for HIPEC in peritoneal metastasis of colorectal cancer:
① Platinum-based chemotherapy drugs: Oxaliplatin. ② Antimetabolite chemotherapy drugs: Raltitrexed. ③ Topoisomerase inhibitors: Irinotecan. ④ Antibiotic chemotherapy drugs: Mitomycin. In addition, biological response modifiers such as recombinant human tumor necrosis factor (rmhTNF) can also be used in combination.
Note:
① In 2003, a phase III prospective clinical study randomly divided patients with peritoneal metastasis of colorectal cancer into two groups: palliative surgery + systemic intravenous chemotherapy (leucovorin/5-fluorouracil) and CRS+HIPEC+systemic intravenous chemotherapy. The median survival times were 12.6 and 22.4 months, respectively.
② At the 9th International Congress on Peritoneal Surface Oncology held in Amsterdam, Netherlands in 2014, PSOGI officially proposed the CRS+HIPEC strategy as the standard treatment for colorectal peritoneal metastasis.
③ National Health and Family Planning Commission of China "China Colorectal Cancer Diagnosis and Treatment Guidelines (2017 edition)": CRS+HIPEC combined with systemic therapy is currently the standard treatment for peritoneal metastasis of colorectal cancer. Systemic therapy includes chemotherapy and/or targeted therapy.
④ In 2020, a study showed that rmhTNF has a direct killing effect on mouse human colon cancer peritoneal tumors at 37℃ and 42℃, and its combination with cisplatin and raltitrexed can significantly promote apoptosis. In addition, a study in China in 2024 showed that intraoperative perfusion of 5 million IU rmhTNF is safe and does not increase the incidence of surgical complications or adverse reactions to chemotherapy drugs, and is significantly effective in treating ascites caused by peritoneal tumors.
2.2 Chemotherapy
After CRS+HIPEC treatment, systemic chemotherapy is indispensable, which can consolidate postoperative treatment, prevent recurrence, and prolong survival. For patients with CCR-0 and CCR-1, adjuvant chemotherapy can be performed; for patients with CCR-2 or CCR-3, palliative chemotherapy should be performed according to advanced colorectal cancer. Recommended adjuvant or palliative chemotherapy regimens include:
(1) First-line chemotherapy regimens
① mFOLFOX6 (2-week cycle): Oxaliplatin 85mg/m² intravenous infusion for 2h d1; Calcium leucovorin 400mg/m² intravenous infusion for 2h d1; Fluorouracil 400mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total amount 2400mg/m² continuous intravenous infusion for 46-48 h).
② FOLFIRI (2-week cycle): Irinotecan 180mg/m² intravenous infusion >30-90min d1; Calcium leucovorin 400mg/m² intravenous infusion for 2h (immediately after irinotecan infusion) d1; Fluorouracil 400mg/m² intravenous bolus d1, 1200mg/(m²·d) continuous intravenous infusion d×2 (total amount 2400mg/m² continuous intravenous infusion for 46-48 h).
③ CAPEOX (3-week cycle): Oxaliplatin 130mg/m² intravenous infusion >2h d1; Capecitabine 1000mg/m² BID oral d1-14.
④ FOLFOXIRI (2-week cycle): Irinotecan 165mg/m² intravenous infusion d1; Oxaliplatin 85mg/m² intravenous infusion d1; Calcium leucovorin 400mg/m² intravenous infusion d1; Fluorouracil total amount 2400-3200mg/m² d1 continuous intravenous infusion for 48 h.
⑤ FOLFIRINOX (2-week cycle): Oxaliplatin 85mg/m² intravenous infusion d1; Calcium leucovorin 400mg/m² intravenous infusion >2h d1; Irinotecan 150mg/m² intravenous infusion >30-90min d1; Fluorouracil 400mg/m² intravenous bolus d1; Fluorouracil 1200 mg/(m²·d) continuous intravenous infusion d×2 (total amount 2400mg/m² continuous intravenous infusion >46 h).
Note: For other chemotherapy regimens and dosages, please refer to the "Principles of Systemic Therapy" in the 2024 NCCN Colorectal Cancer Guidelines.
(2) Second-line chemotherapy regimens
① mFOLFOX6 (2-week cycle) or CAPEOX (3-week cycle): Specific chemotherapy dosages are the same as above, suitable for patients who received irinotecan treatment in the first line.
② FOLFIRI (2-week cycle): Specific chemotherapy dosages are the same as above, suitable for patients who received oxaliplatin treatment in the first line.
③ Oxaliplatin + Raltitrexed (fluorouracil intolerance) (2-week cycle): Oxaliplatin 85g/m² intravenous infusion for 2h d1; Raltitrexed 2mg/m² intravenous infusion for 15min d1.
④ Irinotecan + Raltitrexed (fluorouracil intolerance) (2-week cycle): Irinotecan 180mg/m² intravenous infusion >30-90min d1; Raltitrexed 2mg/m² intravenous infusion for 15min d1.
Note: For other chemotherapy regimens, please refer to the "Principles of Systemic Therapy" in the 2024 NCCN Colorectal Cancer Guidelines.
2.3 Targeted Therapy
(1) First-line treatment regimen
① Bevacizumab injection (Avastin): For primary lesions located in the right colon or KRAS or BRAF mutation type, the 2024 NCCN colorectal cancer guidelines recommend bevacizumab combined with a two-drug chemotherapy regimen (specific chemotherapy dosages are the same as above).
A. 7.5mg/kg intravenous drip d1 (3 weeks per course).
B. 5mg/kg intravenous drip d1 (2 weeks per course).
② Cetuximab (cetuximab, Erbitux): For patients with primary lesions located in the left colon and rectum and with both KRAS and BRAF wild-type, the 2024 NCCN colorectal cancer guidelines recommend cetuximab combined with a two-drug chemotherapy regimen (specific chemotherapy dosage as above).
A. 400mg/m² initial intravenous drip >2h, subsequent 250mg/m² intravenous drip >60min (1 week per course).
B. 500mg/m² d1 intravenous drip >2h (2 weeks per course).
(2) Second-line treatment regimens
① Bevacizumab (Avastin), targeted drug regimen and dosage as before. Suitable for patients with colorectal cancer that has failed first-line chemotherapy, regardless of KRAS and BRAF phenotype, and regardless of whether first-line treatment included cetuximab or bevacizumab chemotherapy.
② Cetuximab (cetuximab, Erbitux), targeted drug regimen and dosage as before. Only applicable to advanced colorectal cancer with both KRAS and BRAF wild-type that did not receive cetuximab in first-line chemotherapy.
③ Vemurafenib (BRAF inhibitor), for patients with RAS wild-type and BRAF V600E mutation, the 2024 NCCN colorectal cancer treatment guidelines recommend vemurafenib + cetuximab + irinotecan or BRAF inhibitor + cetuximab ± MEK inhibitor as second-line and subsequent treatment.
Note: ① If first-line chemotherapy uses chemotherapy combined with cetuximab, it is not recommended to continue using cetuximab in the second-line; if first-line treatment uses chemotherapy combined with bevacizumab, the chemotherapy regimen can be changed in the second-line and continue to be combined with bevacizumab. ② For specific chemotherapy regimens and dosages of other second-line targeted drugs (such as MEK inhibitors, apatinib), please refer to the "General Treatment Principles" in the 2024 NCCN colorectal cancer guidelines.
(3) Third-line treatment regimens
Currently, the NCCN guidelines recommend regorafenib, trifluridine/tipiracil (TAS-102), and fruquintinib as third-line treatment drugs.
① Regorafenib: The multi-target anti-angiogenesis inhibitor regorafenib 160 mg, oral, once daily, continuously for 3 weeks, rest for 1 week, 4 weeks per course.
② Trifluridine/tipiracil: TAS-102 is an oral antimetabolite chemotherapeutic drug. The initial recommended dose is 35mg/m², up to 80 mg, twice daily, d1-5 and d8-12, 4 weeks per course.
③ Fruquintinib: A domestically produced multi-target anti-angiogenesis inhibitor, is a third-line targeted therapy drug for advanced colorectal cancer. Usage: 5mg, oral, once daily, continuously for 3 weeks, rest for 1 week, 4 weeks per course.
④ Trifluridine/tipiracil + Bevacizumab: TAS-102 is 35 mg/m² (maximum single dose is 80mg), up to 80 mg, twice daily, d1-5 and d8-12, 4 weeks per course. Or TAS-102 is 35 mg/m² (maximum single dose is 80mg), up to 80 mg, twice daily, d1-5, 2 weeks per course; bevacizumab 5mg/kg, intravenous drip, d1, 2 weeks per course.
⑤ Cetuximab ± Irinotecan: Specific chemotherapy dosage as above. For patients who have not received cetuximab in the frontline, this regimen is Class 1A evidence; for those who have received cetuximab in the frontline, this regimen is Class 3 evidence.
⑥ HER2 antibodies and inhibitors: For patients with HER2 amplification and RAS and BRAF wild-type, trastuzumab + pertuzumab or trastuzumab + lapatinib are selected as third-line treatment regimens. Based on the results of the DESTINY-CRC01 Phase II study, the anti-HER2 ADC drug, trastuzumab deruxtecan, has shown promising efficacy in the second-line treatment of patients with HER2-overexpressing or amplified advanced colorectal cancer, and it is recommended to conduct Phase III clinical trials to confirm this.
Note: For other third-line targeted monotherapy or combination chemotherapy regimens and dosages, please refer to the "General Treatment Principles" in the 2024 NCCN colorectal cancer guidelines.
2.4 Immunotherapy
Pembrolizumab (PD-1 inhibitor) has been approved for first-line treatment of unresectable or metastatic MSI-H/dMMR colorectal cancer. The 2024 NCCN colorectal cancer guidelines suggest that in addition to MSI-H/dMMR, POLE/POLD1 mutation patients are also sensitive to immunotherapy. For such colorectal cancer patients who did not use immunotherapy in the first-line, second-line and subsequent treatment options may include pembrolizumab and nivolumab, or domestic immunotherapeutic drugs such as envafolimab, toripalimab, tislelizumab, and camrelizumab.
2.5 Radiotherapy
Radiation therapy is mainly used for perioperative treatment, palliative treatment, and integrated treatment of unresectable locally advanced rectal cancer. For patients with local or widespread peritoneal metastasis, if radiation therapy is considered, MDT to HIM discussion and decision-making are required. Radiation therapy techniques and dosages refer to the corresponding sections of the rectal cancer guidelines.
Chapter 4 Treatment of Peritoneal Tumors in 1673 Peritoneal Tumors
Treatment of peritoneal metastasis of ovarian cancer
Satisfactory cytoreduction can be achieved in peritoneal metastasis of ovarian cancer, and CRS surgery should be performed first, followed by integrated treatment with systemic chemotherapy, HIPEC, radiotherapy, etc. If satisfactory cytoreduction surgery cannot be achieved or cannot tolerate surgery, neoadjuvant chemotherapy can be performed first, and the tumor is reassessed after 2-3 cycles. If the tumor achieves remission or stabilization, intermediate cytoreductive surgery (IDS) + HIPEC treatment can be performed, followed by systemic chemotherapy or IDS + systemic chemotherapy, for a total of 6-8 cycles.
Gynecological oncology experts recommend using laparoscopic Fagotti's score to determine whether ovarian cancer patients can receive satisfactory cytoreductive surgery, which is one of the methods to determine whether to perform initial cytoreductive surgery (PDS) or IDS. If Fagotti's score ≥8 points, the possibility of achieving satisfactory CRS is low, and biopsy and neoadjuvant chemotherapy can be considered, followed by IDS.
If Fagotti's score <8 points, the possibility of achieving satisfactory CRS is high, and PDS can be considered.
For ovarian cancer patients who achieve CR or PR after surgery and chemotherapy, maintenance therapy such as bevacizumab, PARP inhibitors, or a combination of PARP inhibitors and bevacizumab can be considered.
3.1 CRS+HIPEC
CRS is the primary surgical treatment for ovarian cancer, which can reduce tumor burden and improve survival benefits. It should aim for macroscopic complete resection, removing as much tumor as possible, with residual lesions ideally less than 1cm in diameter, and preferably within 0.5cm.
The extent of cytoreductive surgery significantly impacts prognosis. If necessary, it may involve the resection of the uterus, bilateral adnexa, parts of the intestines, stomach, spleen, etc., often requiring multi-organ resection and collaboration between the MDT and HIM to achieve satisfactory cytoreduction.
HIPEC is generally performed intraoperatively or postoperatively. Initial cytoreductive surgery combined with HIPEC improves overall survival without increasing the incidence of adverse reactions. Multiple randomized controlled trials suggest that CRS+HIPEC significantly improves the 3-year and 5-year survival rates of patients with stage IIIc/IV and recurrent ovarian cancer, and reduces the risk of primary platinum resistance and malignant bowel obstruction, especially for patients without BRCA mutations. In the absence of contraindications, HIPEC should be considered for all ovarian cancer patients after CRS.
The recommended number of HIPEC treatments for ovarian cancer is 1-3. Chemotherapeutic agents include cisplatin, docetaxel, and paclitaxel. It is important to note that the HIPEC drug regimens and treatment modalities recommended in Western guidelines for ovarian cancer patients are not applicable to Chinese patients. Based on clinical research conducted in China, closed HIPEC is recommended as the first-line treatment for ovarian cancer patients. The maximum dose of cisplatin is 85mg/m², and when paclitaxel is combined with cisplatin HIPEC, the maximum doses of the two drugs are 175mg/m²
and 75mg/m², respectively. If allergic to paclitaxel, docetaxel can be used as a substitute, with a maximum dose of 75mg/m². For patients requiring subsequent bevacizumab treatment, the maximum dose of cisplatin monotherapy HIPEC should not exceed 75mg/m².
Ovarian cancer HIPEC treatment modalities include:
(1) Prophylactic modality
This modality is suitable for ovarian cancer patients with peritoneal seeding. HIPEC after satisfactory cytoreductive surgery can consolidate the surgical effect.
(2) Therapeutic modality
After CRS for ovarian cancer, HIPEC can remove micrometastases and residual lesions, reduce tumor burden, reduce ascites, and alleviate symptoms.
(3) Conversion modality
Suitable for ovarian cancer patients with massive ascites or extensive peritoneal metastasis, who cannot achieve satisfactory cytoreduction or cannot tolerate surgery with PDS. Conversion therapy can be performed with HIPEC combined with systemic chemotherapy to achieve successful conversion, followed by IDS combined with HIPEC treatment.
Note:
①In 2018, the New England Journal of Medicine reported the results of a phase III clinical trial of adding a single HIPEC treatment to patients with residual disease <1cm after neoadjuvant chemotherapy and IDS. Compared with the control group of IDS combined with postoperative intravenous chemotherapy, the median RFS and OS in the IDS+HIPEC plus postoperative intravenous chemotherapy group were extended by 3.5 months and 11.8 months, respectively.
②In 2019, the NCCN guidelines for ovarian cancer included HIPEC in the treatment guidelines after IDS.
③In 2020, a multicenter retrospective clinical study in China showed that HIPEC treatment can improve the 3-year survival rate by 10.5% for patients with stage III ovarian cancer who achieved satisfactory cytoreduction.
④It is recommended to use drug dosages suitable for Chinese patients for HIPEC treatment, including cisplatin monotherapy, cisplatin+paclitaxel, and cisplatin+docetaxel.
3.2 Chemotherapy
(1) First-line chemotherapy regimens for ovarian cancer
1) Paclitaxel + platinum drugs (carboplatin preferred, 6-8 cycles) Paclitaxel 175mg/m² IV infusion 3h d1, carboplatin AUC 5-6 IV infusion 1h d1 (3 weeks per cycle). Paclitaxel 80mg/m² IV infusion 1h d1, 8, 15, carboplatin AUC 5-6 IV infusion 1h d1 (3 weeks per cycle).
2) Docetaxel + platinum drugs (carboplatin preferred, 6-8 cycles) Docetaxel 60-75mg/m² IV infusion 1h d1, carboplatin AUC 5-6 IV infusion 1h d1 (3 weeks per cycle).
3) Liposomal doxorubicin + carboplatin (6 cycles) Pegylated liposomal doxorubicin 30mg/m² IV infusion d1, carboplatin AUC 5 IV infusion 1h d1 (3 weeks per cycle).
Note: The 2024 NCCN ovarian cancer guidelines support the regimen of intraperitoneal paclitaxel chemotherapy, mainly based on the results of the GOG 172 clinical study. This regimen has not been widely used to date, mainly because only 42% of patients completed 6 cycles of intraperitoneal chemotherapy, and the patients' quality of life was poor.
(2) Chemotherapy regimens for platinum-sensitive recurrent ovarian cancer:
1) Platinum-containing chemotherapy regimens: Options include carboplatin + paclitaxel ± bevacizumab, carboplatin + liposomal doxorubicin ± bevacizumab, carboplatin/cisplatin + gemcitabine ± bevacizumab, FOLFOX6/XELOX ± bevacizumab, irinotecan + cisplatin, paclitaxel + nedaplatin, etc.
2) Platinum-free chemotherapy regimens: Primarily single-agent chemotherapy with albumin-bound paclitaxel, capecitabine, cyclophosphamide, liposomal doxorubicin, ifosfamide, oxaliplatin, pemetrexed, vinorelbine, etc.
(3) Chemotherapy regimens for platinum-resistant recurrent ovarian cancer:
Platinum-resistant patients have a poor prognosis, and there are few clinically available chemotherapy regimens, including: liposomal doxorubicin ± bevacizumab, weekly paclitaxel ± bevacizumab, docetaxel, oral VP-16, gemcitabine, oxaliplatin, etc.
Note: For other chemotherapy regimens, please refer to the "General Principles of Systemic Therapy" section of the 2024 NCCN Ovarian Cancer Guidelines.
3.3 Targeted Therapy
(1) Bevacizumab: Patients with advanced ovarian cancer at high risk of recurrence can be treated with bevacizumab (7.5mg/kg or 15mg/kg, intravenous infusion) in combination with chemotherapy. After stopping chemotherapy, continue with bevacizumab (7.5mg/kg or 15mg/kg, intravenous infusion)
maintenance therapy can prolong PFS and OS in high-risk populations.
(2) PARP inhibitors: In patients with advanced epithelial ovarian cancer with BRCA1/2 mutations or HRD positivity, regardless of histology, after platinum-based chemotherapy remission in both newly diagnosed and recurrent patients, PARP inhibitors can be selected for further maintenance therapy, which can significantly prolong PFS in newly diagnosed and platinum-sensitive recurrent ovarian cancer, and has become one of the best choices for targeted therapy. Patients with wild-type BRCA1/2 and HRD negativity can also benefit from PARP inhibitor treatment.
PARP inhibitors: Olaparib (300mg, bid), Talazoparib (60mg, bid), Rucaparib (150mg, bid), and Niraparib (300mg, bid).
(3) Endocrine therapy: Endocrine therapy, including aromatase inhibitors (letrozole, anastrozole, exemestane), tamoxifen, and fulvestrant, can be recommended for patients with biochemically recurrent ovarian cancer.
(4) Others: These mainly include pan-target inhibitors, such as larotrectinib and entrectinib for NTRK gene fusion, trametinib for low-grade serous carcinoma, selpercatinib for RET gene fusion, and dabrafenib and trametinib for BRAFV600E mutation positivity. Sotorasib, the first ADC drug approved by the FDA in 2024 for the treatment of folate receptor α (FRα)-positive, platinum-resistant recurrent ovarian cancer.
Note: For other targeted drugs, please refer to the "General Principles of Systemic Therapy" section of the 2024 NCCN Ovarian Cancer Guidelines.
3.4 Immunotherapy
The results of the DUO-O study in 2023 showed that durvalumab + olaparib + bevacizumab can prolong PFS in patients with BRCA wild-type ovarian cancer, demonstrating for the first time that the addition of immunotherapy to first-line maintenance therapy for ovarian cancer can be beneficial. More phase III RCT studies are needed to confirm this.
3.5 Radiotherapy
Radiotherapy has limited applications in ovarian cancer and is often used as a palliative treatment to relieve symptoms, improve quality of life, and prolong survival.
Radiotherapy can be considered for patients who are inoperable or have chemotherapy resistance. Whole abdominal radiotherapy can be used for peritoneal dissemination. For specific sites, such as unresectable vaginal stumps, cervical lymph nodes, and mediastinal lymph nodes, radiotherapy can help control local lesions.
New radiotherapy techniques such as intensity-modulated radiotherapy, stereotactic radiotherapy, and hypofractionated radiotherapy can help reduce treatment toxicity. MDT to HIM discussion can be conducted before radiotherapy to make decisions.
4. Treatment of PMP
Most PMPs originate from appendiceal mucinous neoplasms and are classified as low-invasive and high-invasive. Low-invasive and mucinous cysts can achieve clinical cure if completely resected surgically without invasion of the serosal layer or rupture. However, tumor rupture, whether low-invasive or high-invasive, is prone to PMP. CRS+HIPEC combined treatment strategy is now the standard treatment for PMP patients.
4.1 CRS+HIPEC
Whether the appendiceal mucinous neoplasm is completely resected is crucial to the efficacy. Surgical treatment must ensure tumor integrity. Tumor perforation or rupture is very easy to spread to the peritoneum and form implantation metastasis. To avoid iatrogenic dissemination caused by surgery, laparoscopic appendectomy is performed, and if a large mucinous neoplasm is found, it is immediately converted to open surgery. If significant peritoneal adhesions or implantation signs are found in the preoperative examination, open surgery can be performed.
If the tumor is intestinal-type appendiceal cancer or poorly differentiated appendiceal mucinous neoplasm, appendiceal lymph node biopsy should be performed. If positive, prophylactic right hemicolectomy should be performed.
HIPEC is extremely important in the treatment of PMP. Chemotherapy drugs used for HIPEC in PMP include: oxaliplatin, mitomycin, cisplatin, docetaxel, and doxorubicin, etc. In recent years, drugs such as tegafur have achieved certain effects in the treatment of peritoneal metastasis of colon cancer, and can also be applied to the treatment of PMP. Currently, the latest recommended HIPEC drug regimen for PMP by the 13th International Peritoneal Cancer Congress is mitomycin combined with cisplatin.
(1) Prophylactic modality
After radical surgery for appendiceal mucinous neoplasms, tissue-level radical cure can be achieved, but improper surgical operation or preoperative rupture and perforation of appendiceal tumor tissue cannot rule out cell-level peritoneal implantation metastasis. HIPEC treatment can be performed to remove free peritoneal cancer cells and subclinical lesions, but further phase III studies are needed to confirm this.
(2) Therapeutic modality
CRS+HIPEC has significant efficacy and can greatly prolong the survival time of some PMP patients. The completeness of CRS is a key factor affecting prognosis. Patients with satisfactory CRS have significantly better prognosis than CCR-2 and CCR-3. Unlike peritoneal metastasis of other digestive tract tumors, even patients with high PCI scores can achieve good prognosis after thorough CRS.
CRS often requires removal of "jelly-like" mucus, but even open abdominal lavage can hardly "wash clean" the abdominal cavity. Improper handling can easily lead to widespread abdominal metastasis. Postoperative standardized combined HIPEC (1-3 times, can be increased to 5 times depending on the situation) treatment can repeatedly and continuously lavage every corner of the abdominal cavity to remove mucus, broken tissue, free cancer cells, and micro-lesions.
Note f:
①In 2012, the J Clin Oncol journal reported the results of the largest clinical study in the world to date. After 2298 cases of PMP were treated with CRS+HIPEC, the 10-year survival rate reached 63%, and the 15-year survival rate reached 59%.
At the 9th International Conference on Peritoneal Surface Oncology held in Amsterdam, Netherlands in 2014, PSOGI officially proposed the CRS+HIPEC strategy as the standard treatment for PMP.
In 2020, PSOGI officially formulated international guidelines for the treatment of PMP with CRS+HIPEC.
Multiple domestic and international consensus recommendations support CRS+HIPEC as the standard treatment for PMP.
4.2 Postoperative Treatment
(1) Postoperative Fluid Management
Because CRS generally involves extensive resection and significant abdominal trauma, resulting in substantial blood and fluid loss and protein loss, the abdominal drainage volume is often high in the first 3 postoperative days. Therefore, postoperative fluid management is crucial for these patients. For PMP patients, the use of relevant precision fluid management tools to achieve individualized fluid therapy, real-time monitoring of fluid intake and output, and dynamic fluid quality management can reduce the risk of cardiovascular adverse events and improve survival.
(2) Postoperative Anti-infection Treatment
Postoperative infection prevention after CRS+HIPEC often requires a combination of multiple antibiotics. A triple regimen is currently recommended: cephalosporins + anaerobic antibiotics + quinolones. The regimen can be adjusted based on the results of bacterial culture and drug sensitivity testing of postoperative abdominal and thoracic drainage fluid. If the drug sensitivity results are not yet available, and Gram-positive bacterial infection is suspected, empirical vancomycin can be considered; if Gram-negative bacterial infection is suspected, empirical levofloxacin can be considered; if fungal infection is suspected, empirical fluconazole can be considered.
(3) Treatment of Postoperative High Myoglobinemia
Prompt intravenous administration of sodium bicarbonate solution is needed. Serum myoglobin levels usually begin to decrease significantly on the second postoperative day and return to normal within 3-4 days. Therefore, if high myoglobinemia occurs in PMP patients postoperatively, sodium bicarbonate can be used to alkalinize the urine, reducing oxidative stress and thus lowering the risk of acute kidney injury.
(4) Prevention and Treatment of Postoperative Venous Thromboembolism
The following measures can be taken to prevent VTE:
Physical Prevention: ① Dorsiflexion-plantar flexion exercises of the ankle joint, 3 times a day, 20 times each time, for 1-10 days postoperatively; ② Upper limb exercises, mainly including chest pump exercises, blowing balloons, and combing hair, 3 times a day, 20 times each time, for 1-10 days postoperatively; ③ Slow walking beside the bed, depending on the patient's postoperative recovery, approximately 3-7 days postoperatively, slow walking or stepping in place 3 times a day, at least 3 minutes each time; 7-10 days postoperatively, 2 times a day, at least 30 minutes each time, depending on the actual situation.
Pharmacological Treatment: If the postoperative D-dimer level decreases and then increases significantly, exceeding 3000 ng/ml DDU, and no deep vein thrombosis is found on lower extremity venous ultrasound, low-molecular-weight heparin 0.3 ml qd can be administered subcutaneously after assessing the absence of bleeding risk; if deep vein thrombosis is found on lower extremity venous ultrasound (intermuscular vein thrombosis in the calf), low-molecular-weight heparin 0.4 ml qd can be administered subcutaneously after assessing the absence of bleeding risk and pulmonary embolism; if DVT is found on lower extremity venous ultrasound (popliteal vein and above vein thrombosis), consultation with a specialist is recommended to consider thrombolysis and other treatments. If there is a bleeding risk or other contraindications to anticoagulation, an inferior vena cava filter can be placed.
4.3 Postoperative Chemotherapy
Systemic chemotherapy for PMP is similar to that for colorectal cancer. For patients receiving CRS+HIPEC, adjuvant chemotherapy before or after surgery is recommended, lasting 6 months. Domestic experts suggest that systemic chemotherapy is suitable for high-grade PMP or patients with lymph
node metastasis, while low-grade patients may not benefit from systemic chemotherapy.
(1) If surgery achieves CC-0/1 and the tumor pathology is peritoneal mucinous carcinomatosis (PMCA) and peritoneal mucinous carcinomatosis with signet ring cells (PMCA-S), chemotherapy based on 5-Fu can be adopted, such as mFOLF-OX6, FOLFIRI, or CAPEOX. The specific chemotherapy regimens are as follows:
1) mFOLFOX6 (2 weeks/cycle): Oxaliplatin 85 g/m² intravenous infusion for 2 h d1; Calcium folinate 400 mg/m² intravenous infusion for 2 h d1; Fluorouracil 400 mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total 2400 mg/m² continuous intravenous infusion for 46-48 h).
2) FOLFIRI (2 weeks/cycle): Irinotecan 180 mg/m² intravenous infusion >30-90 min d1; Calcium folinate 400 mg/m² intravenous infusion for 2 h (immediately after irinotecan infusion) d1; Fluorouracil 400 mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total 2400 mg/m² continuous intravenous infusion for 46-48 h).
3) CAPEOX (3 weeks/cycle): Oxaliplatin 130 mg/m² intravenous infusion >2 h d1; Capecitabine 1000 mg/m² BID oral d1-14.
(2) For CCR-2/3 surgical extent, regardless of pathology, postoperative adjuvant chemotherapy can be attempted, with the same chemotherapy regimens as above. Molecular targeted drugs can also be used in combination, such as bevacizumab. Alternatively, bevacizumab + oxaliplatin + cyclophosphamide combination therapy can be used as a second-line treatment (bevacizumab 7.5 mg/kg intravenous infusion d1, oxaliplatin 130 mg/m² intravenous infusion d1, cyclophosphamide 500 mg/m² intravenous infusion d1, 3 weeks per cycle).
5 Treatment of Hepatobiliary Pancreatic Cancer Peritoneal Metastasis
5.1 CRS+HIPEC
Spontaneous rupture or iatrogenic manipulation of liver cancer can lead to peritoneal metastasis, and the peritoneum is a common site of metastasis for cholangiocarcinoma and pancreatic cancer. Hepatobiliary pancreatic cancer with peritoneal metastasis has a poor overall prognosis and is mainly treated with systemic therapy. For patients with a small tumor burden, after careful selection, integrated treatment with CRS+HIPEC as the main approach can be considered.
(1) Prevention mode: CIS+HIPEC
For patients with hepatobiliary and pancreatic cancer at high risk of peritoneal metastasis, including: ① Liver cancer rupture and bleeding. ② T3, T4 stage tumors. ③ FCC positive. ③ Lymph node metastasis. ④ Accompanied by vascular/lymphatic vessel cancer thrombus, nerve invasion, etc. After radical surgery, preventive HIPEC treatment can be considered for 1-2 times, which can remove intraoperative FCCs and subclinical lesions, reduce the incidence of postoperative peritoneal metastasis, improve long-term survival rate, but further phase III studies are needed to confirm this.
Treatment Mode: CRS+HIPEC
For strictly selected patients with low-volume hepatobiliary and pancreatic cancer peritoneal metastasis, CRS should be performed to remove visible tumors in the abdominal cavity as much as possible under the premise of ensuring surgical safety, maximizing the reduction of tumor burden. When combined with organ resection, gastrectomy, partial small bowel resection, partial colon resection, cholecystectomy, splenectomy, etc. should be performed as needed.
Chemotherapy drugs for HIPEC in peritoneal metastasis of hepatobiliary and pancreatic cancer: cisplatin, oxaliplatin, gemcitabine, mitomycin, etc.
Note:
① In 2018, a multicenter study on biliary tract tumors found that the median survival time for the CRS+HIPEC group and palliative surgery+systemic intravenous chemotherapy group (FOLFOX/FOLFIRI) was 21.4 and 9.3 months, respectively.
② In 2018, a PSOGI multicenter international retrospective study found that the median OS for patients with liver cancer peritoneal metastasis receiving CRS+HIPEC treatment was 46.7 months, and the safety was controllable.
③ In 2021, a domestic single-center retrospective clinical study found that for patients with bile duct cancer peritoneal metastasis, HIPEC treatment can increase the 3-year survival rate from 9.8% to 28.0%.
5.2 Chemotherapy
After CRS+HIPEC treatment, systemic treatment is indispensable, which can consolidate postoperative treatment, prevent recurrence, and prolong survival. For patients with CCR-0 and CCR-1, adjuvant chemotherapy can be performed; for patients with CCR-2 or CCR-3, palliative chemotherapy should be performed according to advanced hepatobiliary and pancreatic cancer. Recommended adjuvant or palliative chemotherapy regimens include:
(1) First-line chemotherapy regimens
1) Primary Liver Cancer
①FOLFOX4 (2-week cycle): Oxaliplatin 85mg/m² intravenous infusion d1; 5-fluorouracil 400mg/m² intravenous infusion d1~2, followed by 600mg/m², continuous intravenous infusion for 22h.
2) Malignant biliary tract tumors
①GC (3-week cycle): Gemcitabine 1000mg/m², cisplatin 25mg/m², intravenous infusion d1, d8.
②GS (3-week cycle): Gemcitabine 1000mg/m², intravenous infusion d1, tegafur 40mg/m², twice daily, oral administration d1~14.
③XELOX (3-week cycle): Oxaliplatin 130mg/m² intravenous infusion d1; capecitabine 1000mg/m², twice daily, oral administration d1~14.
④AG (3-week cycle): Gemcitabine 1000mg/m² intravenous infusion, albumin-bound paclitaxel 125mg/m² d1, d8.
⑤Nal-IRI+5-FU/LV (2-week cycle): Nanoliposomal irinotecan 70 mg/m² d1; calcium folinate 400 mg/m² intravenous infusion 2h d1; 5-fluorouracil 400 mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total 2400 mg/m² continuous intravenous infusion 46~48 h).
3) Pancreatic cancer
①AG (3-week cycle): Gemcitabine 1000mg/m² intravenous infusion, albumin-bound paclitaxel 125mg/m² d1, d8.
②GC (3-week cycle): Gemcitabine 1000mg/m², cisplatin 25mg/m², intravenous infusion d1, d8.
③GX (3-week cycle): Gemcitabine 1000mg/m² intravenous infusion d1, 8; capecitabine 1660mg/m², twice daily, oral administration d1~14.
④GS (3-week cycle): Gemcitabine 1000mg/m², tegafur 40mg/m², twice daily, oral administration d1~14.
⑤FOLFIRINOX (2-week cycle): Oxaliplatin 85mg/m², irinotecan 180mg/m², calcium folinate 400mg/m², 5-fluorouracil 400mg/m² intravenous infusion d1, followed by 5-fluorouracil 2400mg/m², continuous intravenous infusion for 46h.
⑥mFOLFIRINOX (2-week cycle): Oxaliplatin 85mg/m², irinotecan 150mg/m², calcium folinate 400mg/m² intravenous infusion d1, followed by 5-fluorouracil 2400mg/m², continuous intravenous infusion for 46h.
⑦Nal-IRI+5-FU/LV (2-week cycle): Nanoliposomal irinotecan 70 mg/m² d1; calcium folinate 400 mg/m² intravenous infusion 2h d1; 5-fluorouracil 400 mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total 2400 mg/m² continuous intravenous infusion 46~48 h).
Note: For other chemotherapy regimens and dosages, please refer to the "Systemic Treatment Principles" in the 2024 NCCN guidelines for liver cancer, biliary tract tumors, and pancreatic cancer.
(2) Second-line chemotherapy regimens
1) Primary Liver Cancer
There is no standard second-line chemotherapy regimen, and targeted and immune drugs are generally used as substitutes.
2) Malignant biliary tract tumors
①mFOLFOX6 (2-week cycle): Oxaliplatin 85g/m² intravenous infusion 2h d1; calcium folinate 400mg/m² intravenous infusion 2h d1; 5-fluorouracil 400mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total 2400mg/m² continuous intravenous infusion 46~48 h)
②FOLFIRI (2-week cycle): Irinotecan 180mg/m² d1; calcium folinate 400mg/m² intravenous infusion 2h d1; 5-fluorouracil 400mg/m² intravenous bolus d1, 1200 mg/(m²·d) continuous intravenous infusion d×2 (total 2400mg/m² continuous intravenous infusion 46~48 h)
③Nal-IRI+5-FU/LV (2-week regimen): Nanoliposomal irinotecan 70mg/m²d1; Calcium folinate 400mg/m² IV infusion 2h d1; Fluorouracil 400mg/m² IV push d1, 1200 mg/(m²·d) continuous IV infusion d×2 (total 2400mg/m² continuous IV infusion 46~48 h)
3) Pancreatic cancer
①First-line treatment uses gemcitabine-based regimens, while second-line regimens are recommended to be fluorouracil-based, such as FOLFIRINOX, CAPEOX, etc.
②First-line treatment uses fluorouracil-based regimens, while second-line regimens are recommended to be gemcitabine-based, such as AG, GP, GX, etc.
③Nal-IRI+5-FU/LV (2-week regimen): Specific dosage and usage are the same as above.
Note: For other chemotherapy regimens and dosages, please refer to the "General Treatment Principles" in the 2024 NCCN Guidelines for Liver Cancer, Biliary Tract Tumors, and Pancreatic Cancer. 1681 Peritoneal Tumor Chapter 4 Treatment of Peritoneal Tumors
5.3 Targeted Therapy
(1) First-line treatment regimen
1) Primary Liver Cancer
①Bevacizumab injection (Avastin): Bevacizumab combined with immune checkpoint inhibitors (including atezolizumab, sintilimab) are first-line treatment options for primary liver cancer.
②Multi-target receptor tyrosine kinase inhibitors: Multi-target receptor tyrosine kinase inhibitors are recommended as first-line regimens, with options including donafenib, lenvatinib, sorafenib, apatinib + camrelizumab, etc.
2) Biliary Tract Tumors and Pancreatic Cancer
①For biliary tract and pancreatic tumors with NTRK gene fusion positivity, larotrectinib or entrectinib are recommended as first-line treatment. For biliary tract and pancreatic malignancies with specific gene mutations (such as ALK gene rearrangement, HER2 amplification, RET fusion, BRAF V600E mutation), etc., the corresponding targeted therapy has a certain effect. These patients are recommended to participate in corresponding clinical studies, and treatment with special targeted drugs can also be considered.
②For KRAS wild-type, based on the results of the NOTABLE study, gemcitabine + nivolumab is recommended; for KRAS mutant type, this regimen is a Class II recommendation.
③For patients with BRCA1/2 mutations who have not shown disease progression after more than 16 weeks of platinum-containing chemotherapy, olaparib maintenance therapy can be recommended.
(2) Second-line treatment regimens
1) Primary Liver Cancer
Options include regorafenib, apatinib, remusimumab (serum AFP level ≥400μg/L), cabozantinib.
2) Biliary Tract Tumors
Options include regorafenib; for BRAF V600E mutation, dabrafenib + trametinib is recommended; for IDH1 mutation, enasidenib is recommended; for FGFR2 fusion/rearrangement, pemigatinib is optional; for HER2 positive, tucatinib or pertuzumab + trastuzumab is recommended; for RET fusion, pralsetinib/selumetinib is recommended.
3) Pancreatic cancer
For KRAS G12C mutation, adagrasib and sotorasib are optional.
For patients who received first-line treatment with combination immunotherapy, single immunotherapy, or single tyrosine kinase inhibitors, second-line regimens can be selected based on disease progression and the specific first-line regimen, choosing approved second-line drugs or previously unused first-line drugs.
5.4 Immunotherapy
Immunotherapy has become an important cornerstone in treatment regimens, and dual immunotherapy has also made significant progress in liver cancer treatment.
Anti-angiogenic drugs combined with immunotherapy, targeted drugs and/or combination immunotherapy have become important treatment methods for unresectable or advanced liver and biliary tract tumors, and are also important means of converting treatment for liver and biliary tract tumors. Immunotherapy with immune checkpoint inhibitors also has a certain effect on some advanced pancreatic cancers.
Using TACE combined with targeted and immunotherapy can prolong the survival time of patients with advanced liver cancer, and the effect is better than single TACE treatment. The CHANCE001 study showed that combined treatment significantly improved the prognosis of patients with advanced liver cancer compared to TACE alone.
Dual immunotherapy has also achieved success in advanced liver cancer. The HIMALAYA study showed that the PD-L1 inhibitor durvalumab + CTLA-4 inhibitor tremelimumab (STRIDE regimen) can reduce the risk of death by 29% in Asian populations, and the risk of death in HBV-positive patients decreased by 34%. This regimen has been included in the NCCN and EMSO guidelines, but has not been approved in China.
The TOPAZ-1 and KEYNOTE-966 clinical trials showed that immunotherapy combined with GC regimens can improve OS in advanced biliary tract tumors and has been approved for first-line treatment.
5.5 Radiotherapy
Radiotherapy is mainly used for perioperative treatment, palliative treatment, and integrated treatment of locally advanced liver, biliary tract, and pancreatic cancer. For patients with local or widespread peritoneal metastases, if radiotherapy is considered, MDT toHIM discussion and decision-making are required.
Section 4 Other Treatments for Peritoneal Tumors
1. Biological Therapy
Biological therapy is a method of using the immune system to treat cancer, such as cytokines, immune cells, monoclonal antibodies, and gene recombination technology, by activating or enhancing the body's immune response to attack and destroy tumor cells.
The field of tumor biological therapy is very broad, mainly divided into:
(1) Non-specific immunotherapy
1) One type is achieved by directly stimulating cytokines, such as IL-2, α-interferon, and tumor necrosis factor (TNF). Currently, there is limited evidence, and it is only recommended for clinical research.
2) Another type works by inhibiting the immune negative regulation process, but further clinical research is needed.
(2) Adoptive immunotherapy
Cells used in adoptive immunotherapy can be derived from blood, tumor tissue, metastatic lymph nodes, or malignant ascites, including lymphokine-activated killer cells (LAK), dendritic cell-activated cytokine-induced killer cells (D-CIK), chimeric antigen receptor-modified T cells (CAR-T), and tumor-infiltrating lymphocytes (TIL). Currently in the experimental stage, these treatments are expensive, lack standardized regulation, and show significant variations in clinical efficacy. National policies are required to allow clinical promotion and application. Chapter 4, Treatment of Peritoneal Tumors, 1683 Peritoneal Tumors
In the field of adoptive immunotherapy for peritoneal tumors, CAR-T cell therapy has demonstrated superior tumor-suppressing activity. Due to the high expression of mesothelin in malignant mesothelioma, CAR-T targeting mesothelin is currently undergoing Phase I/II clinical trials. CEA CAR-T targeting digestive tumor peritoneal metastasis also shows promise. Dual-target CAR-T cell therapy may help better address peritoneal tumor antigen escape and warrants further research.
2. Traditional Chinese Medicine (TCM) Treatment
Traditional Chinese Medicine plays an important auxiliary role in improving the physical condition of peritoneal tumor patients, enhancing immunity, improving quality of life, reducing tumor treatment-related complications, stabilizing tumors, and preventing and treating post-surgical recurrence. TCM treatment follows a holistic approach, primarily focusing on syndrome differentiation and treatment, with disease differentiation as a secondary consideration. It emphasizes the combination of syndrome and disease differentiation, local and holistic perspectives, and a comprehensive treatment system that supports the body's positive energy and eliminates pathogenic factors.
Disease Differentiation Treatment
Disease differentiation treatment is an important method in TCM treatment. Based on the clinical manifestations and pathogenesis of peritoneal tumors, a basic prescription is formulated, and adjustments are made according to symptoms.
(1) Constipation
1) Internal Treatment: The basic treatment method is to promote bowel movements. Basic prescription: rhubarb, Citrus aurantium, Magnolia officinalis, Glauber's salt, Raphanus sativus, etc.; Clinical adjustments: abdominal distension and pain, Qi stagnation add Chuanxiong, Costus, Aucklandia, etc.; shortness of breath and fatigue, Qi and blood deficiency add Astragalus, Angelica, donkey-hide gelatin, and Physalis, etc.; five heart irritability, yin deficiency and dryness add Scrophularia, Ophiopogon, and Radix Rehmanniae, etc. Patent medicines: Mare's milk soft capsules, Mare's milk nourishing spleen pills; Citrus aurantium pills to remove stagnation, Mojia Qingning pills; Aloe capsules, Tongbianling.
2) External Treatment: Acupuncture at Neiguan, Hegu, Zusanli, Shangjuhu, Xiajuhu, etc.; ear acupuncture: large intestine, rectum, sympathetic, etc.; acupoint massage: Zusanli, Zhongwan, Liangmen, Tianshu, etc.
(2) Abdominal Distension
1) Internal Treatment: The basic treatment method is to regulate Qi, invigorate the spleen, and eliminate distension. Basic prescription: Evodia rutaecarpa, Raphanus sativus, Magnolia officinalis, Cyperus rotundus, Aucklandia, Citrus aurantium, etc.; Clinical adjustments: abdominal distension with constipation and full bowels, add rhubarb, Citrus aurantium, etc.; indigestion, frequent hiccups, add Inula, Haematite, fried Hawthorn, fried Malt, cloves, and Persimmon calyx, etc.
Patent medicines: Bupleurum Liver-regulating pills, Agarwood soothing stagnation tablets, Citrus aurantium and Atractylodes pills, Six-flavor Anxiao powder, etc.
2) External Treatment: Acupuncture at Waiguan, Hegu, Yanglingquan, Zusanli, Taichong, etc.; moxibustion at Shenque, Tianshu, Zhongwan, etc.; ear acupuncture: stomach, liver, sympathetic, subcortex, etc.
(3) Nausea and Vomiting
1) Internal Treatment: The basic treatment method is to harmonize the stomach, descend Qi, and stop vomiting. Basic prescription: Pinellia, ginger, Citrus peel, Inula, Haematite, Bambusa, etc.; Clinical adjustments: abdominal distension, heartburn, and upward flow of stomach Qi, add malt, chicken gizzard, Raphanus sativus, and cuttlefish bone, etc.; abdominal distension and pain, Qi stagnation, add Citrus aurantium, Amomum, Corydalis, Evodia rutaecarpa, Cyperus rotundus, and Curcuma, etc.
Patent medicines: Yueju Baohuo pills, Lizhong pills, Gastrointestinal An, etc.
2) External Treatment: Acupuncture at Zan Zhu, Neiguan, Hegu, Ge Yu, Yanglingquan, Taichong, etc.; moxibustion at Shenque, Zusanli, Zhongwan, etc.; acupoint application: Shenque, Shangwan, Zhongwan, Zusanli, etc.; ear acupuncture: spleen, stomach, sympathetic, Shenmen, etc.
(4) Cancer-related Pain and Fatigue
TCM treatments such as acupoint massage and ear seed pressing can help relieve cancer pain. The 2023 NCCN guidelines for cancer-related fatigue suggest that acupuncture and acupoint massage (including infrared laser moxibustion and transcutaneous acupoint stimulation) have some efficacy in recovering from cancer-related fatigue, but due to limited and heterogeneous data, it is difficult to clearly evaluate their benefits. Traditional Chinese medicine plays an important auxiliary role in cancer prevention and treatment, but its clinical application potential in peritoneal tumor treatment requires further research.
3. Nutritional Support
Patients with peritoneal malignant tumors often exhibit malnutrition. Nutritional therapy should select appropriate routes and methods based on the patient's condition and gastrointestinal function. If patients can orally ingest 2/3 of their nutritional needs, oral nutritional supplementation can be used; otherwise, enteral tube feeding is required. If nutrients cannot be ingested, digested, and absorbed through the gastrointestinal tract, total parenteral nutrition (TPN) should be given.
During HIPEC treatment, patients are in a state of stress, and their metabolism is in negative nitrogen balance, requiring high nutritional support. High-protein, high-calorie, low-sugar diets should be provided with appropriate nutritional support, such as TPN, parenteral nutrition, and enteral nutrition, along with glutamine and arginine preparations.
The 2024 NCCN guidelines for gastric cancer recommend establishing appropriate follow-up for patients at potential nutritional risk for lifelong monitoring and management, including but not limited to deficiencies in vitamin B12, iron, zinc, calcium, and vitamin D. Regular daily supplementation with multivitamins/minerals, vitamin B12, calcium, and vitamin D may be considered.
The 2023 NCCN guidelines for cancer-related fatigue recommend providing nutritional counseling to address nutritional deficiencies caused by factors such as anorexia, diarrhea, nausea, and vomiting, which helps patients manage their nutritional status. A high-fiber, low-fat diet rich in fruits, vegetables, whole grains, and foods high in omega-3 polyunsaturated fatty acids can help improve cancer-related fatigue. It is recommended to consult or refer to a nutritionist for nutritional consultation and intervention.
There is no unified standard for nutritional support for patients undergoing HIPEC treatment, and further exploration is needed.
4 Multidisciplinary Integrated Treatment
Peritoneal tumors can originate from different organs in the abdominal cavity, and clinical manifestations lack specificity. A single department cannot accurately diagnose them. An individualized integrated treatment plan needs to be developed for patients through MDT to HIM. Patients' conditions are complex, and most are already in the late stages when they seek medical attention, making radical surgery impossible. Treatment methods from various departments should be integrated in a planned and reasonable manner, based on the patient's physical condition, tumor pathology, invasion range, and development trend. Treatment plans vary greatly for peritoneal tumors of different origins. The MDT to HIM model allows for a deeper understanding of the patient's condition and the development of a more comprehensive integrated treatment plan for cancer patients.
1685 Peritoneal Tumors Chapter 4 Treatment of Peritoneal Tumors
Section 5: Complications of CRS combined with HIPEC
CRS complications are mainly related to the patient's own condition, PCI index, the technical level of the surgical team, and the use of postoperative drugs. Intraoperative complications mainly include organ damage and vascular damage. Digestive and urinary systems are most easily affected by organ damage. The anterior rectal wall is the most frequently damaged part of the digestive system, as it is the lowest point in the pelvis, with limited surgical space, making it prone to avulsion injuries. Damage to the duodenum, jejunum, ileum, and colon is mostly mechanical damage caused by operational errors. Urinary system damage most often involves the bladder and ureters. The most obvious finding is uncontrollable watery bleeding during surgery. Vascular damage is also common. Tumor invasion of the vascular adventitia or vascular variations producing new branches can easily lead to vascular damage. In the hematopoietic system, a few patients may experience myelosuppression such as leukopenia.
CRS+HIPEC combined treatment has a relatively small impact on abdominal organs. Some patients experience complications such as anorexia, abdominal distension, and abdominal pain, which generally recover quickly after treatment and removal of the abdominal perfusion tube. In a few patients, gastrointestinal function has not significantly improved, mainly related to their own diseases and surgical factors. HIPEC does not increase the risk of anastomotic leakage, which is mainly related to the patient's nutritional status, surgical skills, anastomotic tension, and blood supply.
Section 6: Efficacy Evaluation of CRS combined with HIPEC
HIPEC has made continuous breakthroughs in theoretical research and technology, and has become an effective adjuvant treatment for peritoneal tumors, attracting widespread attention from scholars at home and abroad. HIPEC has a unique therapeutic effect in treating primary peritoneal tumors and secondary peritoneal tumors such as gastric cancer, colorectal cancer, ovarian cancer, and appendiceal mucinous tumors, as well as the associated malignant ascites, significantly improving quality of life and long-term survival rates.
At the 9th International Congress on Peritoneal Surface Oncology in 2014, PSOGI officially proposed the CRS+HIPEC strategy as the standard treatment for PMP, peritoneal metastasis of colorectal cancer, and MPM; and as a recommended treatment for peritoneal metastasis of ovarian cancer and gastric cancer. The 2019 NCCN Guidelines for Ovarian Cancer included HIPEC in the guidelines for post-IDS treatment.
The 2021 NCCN Guidelines for Gastric Cancer added HIPEC content: HIPEC or laparoscopy-assisted HIPEC may be a treatment option for carefully selected stage IV patients. The 2024 NCCN Guidelines for Gastric Cancer suggest that HIPEC may be effective for carefully selected patients with low-volume peritoneal metastasis, but further clinical research is needed. Currently, many institutions in China are conducting multicenter randomized controlled trials of CRS combined with HIPEC for the treatment of peritoneal tumors, and the preliminary results are encouraging.
Chapter 5: Clinical Follow-up and Prognosis
Section 1: Follow-up of Peritoneal Tumors
After comprehensive and detailed treatment, regular check-ups should be performed to closely monitor the patient's condition. If the condition progresses, timely treatment and changes to the treatment plan should be implemented. After completion of treatment for peritoneal tumors, regular and standardized examinations should be performed on schedule.
Within the first year, a follow-up examination should be performed every month. Within the second year, if the condition does not progress, the interval can be extended to 2-3 months. From the third to fifth year, a follow-up examination should be performed every 6 months. After 5 years, the interval can be extended to every 12 months, depending on the specific situation.
During regular follow-up, if the condition progresses, monthly follow-up examinations should be resumed. Each follow-up visit should include a detailed record of the patient's condition. If the treatment is effective, the original plan can be maintained; if the condition progresses, the plan should be changed promptly, and the effectiveness of the subsequent treatment plan should be evaluated.
A physical examination should be performed at each return visit. Secondary peritoneal tumors can metastasize to lymph nodes, and physical examination can detect some enlarged distant lymph nodes.
(1) Serum Testing
CA125 has become a routine and effective test for primary peritoneal tumors. It is also elevated in abdominal tuberculosis, making differentiation somewhat difficult. However, in tuberculosis patients, CA125 is generally below 50 ng/L, while it is significantly elevated in primary peritoneal tumors, and the expression level is positively correlated with the degree of peritoneal tumor dissemination. For secondary peritoneal tumors, there are more serum markers, and CA125, CA199, CEA, AFP, CA724, and HCG are all closely monitored indicators.
(2) Imaging Examination
Ultrasound, CT, MRI, and PET/CT are routine examination items for peritoneal tumors.
Ultrasound can detect ascites and perform ascites localization puncture and drainage. It can also detect hypoechoic nodules in the peritoneum, but it is easily affected by surrounding organs and tissues.
CT can clearly show the overall positional relationship between the tumor and surrounding tissues, as well as the proximity of important blood vessels.
MRI has better resolution of malignant nodules in the abdominal cavity and their surrounding soft tissues.
PET/CT can detect small lesions through metabolic enhancement of the lesion and can detect small lesions that cannot be detected by other imaging methods, playing an important role in detecting distant metastasis.
Section 2: Prognosis of Peritoneal Tumors
Peritoneal tumors have a poor overall prognosis, and prevention is key. Early intervention after radical surgery for gastric cancer, colorectal cancer, ovarian cancer, appendiceal mucinous tumors, etc., focusing on preventing peritoneal metastasis and improving cure rates, is a key breakthrough direction. Early detection, early diagnosis, and standardized treatment are crucial for achieving satisfactory clinical efficacy. Whether peritoneal tumors can undergo satisfactory surgical treatment and standardized HIPEC is an important factor affecting the effectiveness of CRS+HIPEC. With further research into its pathogenesis and related treatments, the prognosis of peritoneal tumors has been significantly improved.
1688 China Integrated Oncology Treatment Guidelines
References
[1]Han B,Zheng R,Zeng H,et al.Cancer incidence and mortality in China,2022[J].J Natl Cancer Cent.2024;4(1):page.
[2]Bray F,Laversanne M,Sung H,et al.Global cancer statistics 2022:GLOBOCAN estimates of inci⁃
dence and mortality worldwide for 36 cancers in 185 countries[J].CA Cancer J Clin.2024;74(3):229-263.
[3]Fan Daiming.Integrated Oncology Clinical Volume[M].Beijing: Science Press, 2021.
[4]Cui Shuzhong.Celiotomy hyperthermic perfusion treatment[M].Beijing: People’s Medical Publishing House, 2021.
[5]Fan Daiming, Cui Shuzhong.China Integrated Oncology Treatment Guidelines (CACA) Peritoneal Tumors[M].Tianjin: Tianjin Science and Technology Press, 2022.
[6]Fan Daiming, Cui Shuzhong.China Integrated Oncology Technology Guidelines (CACA) C-HIPEC Technology[M].Tianjin: Tianjin Science and Technology Press, 2023.
[7]Fan Daiming, Xu Huimian.China Integrated Oncology Treatment Guidelines (CACA) Gastric Cancer[M].Tianjin: Tianjin Science and Technology Press, 2022.
[8]Fan Daiming, Wang Xishan.China Integrated Oncology Treatment Guidelines (CACA) Colorectal Cancer, Anal Cancer[M].Tianjin: Tianjin Science and Technology Press, 2022.
[9]Lei Z,Wang J,Li Z,et al.Hyperthermic intraperitoneal chemotherapy for gastric cancer with peritone⁃al metastasis:A multicenter propensity score-matched cohort study[J].Chin J Cancer Res,2020,32(6):794-803.
[10]Guan Tianpei, Lei Ziying, Cui Shuzhong.Clinical Research on Prevention and Treatment of Peritoneal Metastasis of Colorectal Cancer[J].Surgical Theory and Practice, 2021, 26(01): 7-10.
[11]Lheureux S,Gourley C,Vergote I,et al.Epithelial ovarian cancer[J].Lancet,2019,393(10177):1240-1253.
[12]Chua T C,Moran B J,Sugarbaker P H,et al.Early-and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hy⁃perthermic intraperitoneal chemotherapy[J].J Clin Oncol,2012,30(20):2449-2456.
[13]McKenney J K,Gilks C B,Kalloger S,et al.Classification of Extraovarian Implants in Patients With Ovarian Serous Borderline Tumors(Tumors of Low Malignant Potential)Based on Clinical Outcome[J].Am J Surg Pathol,2016,40(9):1155-1164.
[14]Spirtas R,Heineman E F,Bernstein L,et al.Malignant mesothelioma:attributable risk of asbestos exposure[J].Occup Environ Med,1994,51(12):804-811.
[15]Strauss D C,Hayes A J,Thomas J M.Retroperitoneal tumours:review of management[J].Ann R Coll Surg Engl,2011,93(4):275-280.
[16]Pascual-Anton L,Cardenes B,Sainz D L C R,et al.Mesothelial-to-Mesenchymal Transition and Exosomes in Peritoneal Metastasis of Ovarian Cancer[J].Int J Mol Sci,2021,22(21).
[17]Mikula-Pietrasik J,Uruski P,Tykarski A,et al.The peritoneal"soil"for a cancerous"seed":a com⁃prehensive review of the pathogenesis of intraperitoneal cancer metastases[J].Cell Mol Life Sci,2018,
75(3):509-525.[18]Spratt J S,Adcock R A,Muskovin M,et al.Clinical delivery system for intraperitoneal hyperthermic chemotherapy[J].Cancer Res,1980,40(2):256-260.
[19]Fan Daiming.Integrated Oncology Basic Volume[M].Xi’an: World Book Publishing Xi’an Co., Ltd., 2021.
[20]China Anti-Cancer Association Peritoneal Tumor Professional Committee.Expert Consensus on Clinical Application of Celiotomy Hyperthermic Perfusion Chemotherapy Technology in China (2019 Edition)[J].Chinese Medical Journal, 2020(02): 89-90.
[21]China Anti-Cancer Association Peritoneal Tumor Professional Committee, China Anti-Cancer Association Tumor Hyperthermia Professional Committee, Beijing Cancer Prevention and Treatment Association Tumor
Hyperthermia Professional Committee.Chinese Expert Consensus on Diagnosis and Treatment of Diffuse Malignant Peritoneal Mesothelioma[J].Chinese Medical Journal, 2021, 101 1689 Peritoneal Tumor References (36): 2839-2849.
[22]Deraco M,Yang Zhiran.Advances in the treatment of malignant peritoneal mesothelioma at the Milan National Cancer Center[J].Chinese Journal of Oncology Clinical, 2022, 49(24): 1295-1298.
[23]Kusamura S,Kepenekian V,Villeneuve L,et al.Peritoneal mesothelioma:PSOGI/EURACAN clinical practice guidelines for diagnosis,treatment and follow-up[J].Eur J Surg Oncol.2021;47(1):36-59.
[24]Chen Wanqing, Li Ni, Lan Ping, et al.Chinese Guidelines for Screening, Early Diagnosis and Treatment of Colorectal Cancer (2020, Beijing)[J].Chinese Journal of Oncology, 2021, 30(01): 1-28.
[25]Zwanenburg ES,El Klaver C,Wisselink DD,et al.Adjuvant Hyperthermic Intraperitoneal Chemotherapy in Patients With Locally Advanced Colon Cancer(COLOPEC):5-Year Results of a Randomized Multicenter Trial[J].J Clin Oncol.2024;42(2):140-145.
[26]Rau B,Lang H,Koenigsrainer A, et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cyto-reductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GAS-TRIPEC-I Trial[J].J Clin Oncol.2024;42(2):146-156.
[27]Li Jing, Wu Miaofang, Lin Zhongqiu. 《FIGO 2018妇癌报告》——卵巢癌、输卵管癌、腹膜癌诊治指南解读[J]. 中国实用妇科与产科杂志,2019,35(03):304-314.
[28]McCluggage W G,Judge M J,Clarke B A, et al. Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the International Collaboration on Cancer Reporting(ICCR)[J].Mod Pathol,2015,28(8):1101-1122.
[29]Roushdy-Hammady I,Siegel J,Emri S, et al. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey[J].Lancet,2001,357(9254):444-445.
[30]Glehen O,Passot G,Villeneuve L, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study[J].BMC Cancer,2014,14:183.
[31]Pei Wei, Xiong Bin, Cui Shuzhong, et al. 结直肠癌腹膜转移预防和治疗腹腔用药中国专家共识(Ⅴ2019)[J].中华结直肠疾病电子杂志,2019,8(04):329-335.
[32]Li Yan, Xu Hongbin, Peng Zheng, et al. 肿瘤细胞减灭术加腹腔热灌注化疗治疗腹膜假黏液瘤专家共识[J].中华医学杂志,2019(20):1527-1535.
[33]Kim S J,Kim H H,Kim Y H, et al. Peritoneal metastasis: detection with 16-or 64-detector row CT in patients undergoing surgery for gastric cancer[J].Radiology,2009,253(2):407-415.
[34]Bozkurt M,Doganay S,Kantarci M, et al. Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values[J].Eur J Radiol,2011,80(2):224-228.
[35]Low R N,Sebrechts C P,Barone R M, et al. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study[J].AJR Am J Roentgenol,2009,193(2):461-470.
[36]Dromain C,Leboulleux S,Auperin A, et al. Staging of peritoneal carcinomatosis: enhanced CT vs.PET/CT[J].Abdom Imaging,2008,33(1):87-93.
[37]Hu J,Zhang K,Yan Y, et al. Diagnostic accuracy of preoperative(18)F-FDG PET or PET/CT in detecting pelvic and para-aortic lymph node metastasis in patients with endometrial cancer: a systematic review and meta-analysis[J].Arch Gynecol Obstet,2019,300(3):519-529.
[38]Schmeler K M,Sun C C,Malpica A, et al. Low-grade serous primary peritoneal carcinoma[J].Gynecol Oncol,2011,121(3):482-486.
[39]Zuo T,Wong S,Buza N, et al. KRAS mutation of extraovarian implants of serous borderline tumor: prognostic indicator for adverse clinical outcome[J].Mod Pathol,2018,31(2):350-357.
[40]Dilani Lokuhetty V A W M. WHO Calssification of tumor(5th Edition)Female Genital Tumors[M].International Agency for Research,2020.
[41]Umetsu SE,Kakar S. Staging of appendiceal mucinous neoplasms: challenges and recent updates[J].Hum Pathol.2023,132:65-76.
[42]Yuan Z,Chen W,Liu D, et al. Peritoneal cell-free DNA as a sensitive biomarker for detection of peritoneal metastasis in colorectal cancer: a prospective diagnostic study: A prospective diagnostic study[J].Clin Epigenetics.2023;15(1):65.
[43]Gege Z,Xueju W,Bin J. Head-To-Head Comparison of 68Ga-FAPI PET/CT and FDG PET/CT for the Detection of Peritoneal Metastases: Systematic Review and Meta-Analysis.AJR Am J Roentgenol[J].2023;220(4):490-498.
[44]Fu C,Zhang B,Guo T, et al. Imaging Evaluation of Peritoneal Metastasis: Current and Promising Techniques[J].Korean J Radiol.2024;25(1):86-102.
[45]Dong D,Tang L,Li ZY, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer[J].Ann Oncol.2019;30(3):431-438.
[46]Liu S,He J,Liu S, et al. Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer[J].Eur Radiol.2020;30(1):239-246.
[47]Huang W,Zhou K,Jiang Y, et al. Radiomics Nomogram for Prediction of Peritoneal Metastasis in Patients With Gastric Cancer[J].Front Oncol.2020;10:1416.
[48]Liu Tonghua. Liu Tonghua Diagnostic Pathology[M]. Beijing: People's Medical Publishing House, 2018.
[49]Qu Yanjun, Zhao Xiaoyang, Dong Lina. Ultrasound diagnosis of ovarian cancer peritoneal and greater omentum metastasis[J]. Chinese Medical Imaging Technology, 2010, 26(07): 1334-1336.
[50]Ding Ping'an, Liu Yang, Guo Honghai, et al. Application of laparoscopic exploration combined with peritoneal shedding cytology in the diagnosis and treatment of locally advanced gastric cancer. Chinese Journal of Gastrointestinal Surgery, 2020, 23(02): 170-176.
[51]Burbidge S,Mahady K,Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer[J].Clin Radiol,2013,68(3):251-255.
[51]Burbidge S,Mahady K,Naik K.The role of CT and staging laparoscopy in the staging of gastric cancer[J].Clin Radiol,2013,68(3):251-255.
Van T S I, Engbersen M P, Bhairosing P A, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis[J]. Eur Radiol, 2020, 30(6): 3101-3112.
Kim S J, Lee S W. Diagnostic accuracy of (18)F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis[J]. Br J Radiol, 2018, 91(1081): 20170519.
Valasek M A, Pai R K. An Update on the Diagnosis, Grading, and Staging of Appendiceal Mucinous Neoplasms[J]. Adv Anat Pathol, 2018, 25(1): 38-60.
Yan TD, Deraco M, Elias D, et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer[J]. 2011; 117(9): 1855-1863.
Cascales-Campos P A, Gil J, Gil E, et al. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer[J]. Ann Surg Oncol, 2014, 21(7): 2383-2389.
Paul H. Sugarbaker. Cytoreductive surgery and perioperative chemotherapy for peritoneal surface tumors[M]. Translated by Li Yan, Beijing: Science Press, 2018.
Feldman A L, Libutti S K, Pingpank J F, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy[J]. J Clin Oncol, 2003, 21(24): 4560-4567.
Ceelen W P, Flessner M F. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence[J]. Nat Rev Clin Oncol, 2010, 7(2): 108-115.
evidence[J]. Nat Rev Clin Oncol, 2010, 7(2): 108-115.
Yan T D, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience[J]. J Clin Oncol, 2009, 27(36): 6237-6242.
Yang Zhiran, Su Yandong, Yang Rui, et al. Analysis of complications and risk factors of cytoreductive surgery combined with intraperitoneal hyperthermic perfusion in the treatment of malignant peritoneal mesothelioma[J]. Chinese Journal of Oncology Clinical, 2023, 50(13): 661-666.
Helm J H, Miura J T, Glenn J A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis[J]. Ann Surg Oncol, 2015, 22(5): 1686-1693.
Lambert L A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis[J]. CA Cancer J Clin, 2015, 65(4): 284-298.
Vogelzang N J, Rusthoven J J, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma[J]. J Clin Oncol, 2003, 21(14): 2636-2644.
Baas P, Scherpereel A, Nowak A K, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial[J]. Lancet, 2021, 397(10272): 375-386.
Sugarbaker P H. Prevention and Treatment of Peritoneal Metastases from Gastric Cancer[J]. J Clin Med, 2021, 10(9).
Hu H, Zhao J, Yuan J, Zhang M. Peripheral PD-1 and Tim-3 percentages are associated with primary sites and pathological types of peritoneal neoplasms[J]. BMC Cancer. 2023; 23(1): 287.
Hu H, Zhang M. Correlation analysis between peripheral blood dendritic cell subsets and PD-1 in patients with peritoneal adenocarcinoma[J]. Braz J Med Biol Res. 2024; 57: e13192.
Patricia J. Eifel, M.D., Professor. Role of radiation therapy[J]. Best Practice & Research Clinical Obstetrics and Gynaecology. 2017, 41: 118-125.
Chan JK, Teoh D, Hu JM, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers[J]. Gynecol Oncol. 2008; 109(3): 370-376.
Chan JK, Tian C, Fleming GF, et al. The potential benefit of 6 vs. 3 cycles of chemotherapy in subsets of women with early-stage high-risk epithelial ovarian cancer: an exploratory analysis of a Gynecologic Oncology Group study[J]. Gynecol Oncol. 2010; 116(3): 301-306.
Chinese Anti-Cancer Association Gastric Cancer Professional Committee. Chinese expert consensus on the diagnosis and treatment of gastric cancer peritoneal metastasis (2023 edition). Chinese Journal of Gastrointestinal Surgery, 2023, 26(08): 717-728.
Yu Tao, Ma Fuhai, An Qi, et al. Safety and efficacy of prophylactic intraperitoneal hyperthermic perfusion chemotherapy in elderly patients with locally advanced gastric cancer based on propensity score matching analysis. Chinese Medical Journal, 2023, 103(36): 2867-2873.
Ding Pingan, Yang Peigang, Tian Yuan, et al. Efficacy of intraperitoneal hyperthermic perfusion chemotherapy combined with systemic chemotherapy and apatinib conversion therapy for gastric cancer peritoneal metastasis[J]. Chinese Journal of Oncology Clinical, 2021, 48(08): 409-414.
Ding Pingan, Yang Peigang, Guo Honghai, et al. Safety analysis of abdominal wall chemotherapy port in the application of NIPS chemotherapy for gastric cancer peritoneal metastasis[J]. Chinese Journal of General Surgery, 2021, 30(10): 1151-1159.
Ba M, Cui S, Long H, et al. Safety and Effectiveness of High-Precision Hyperthermic Intraperitoneal Perfusion Chemotherapy in Peritoneal Carcinomatosis: A Real-World Study[J]. Front Oncol, 2021, 11: 674915.
Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575-1581.
Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37(23):2028-2040.
Newhook TE, Agnes A, Blum M, et al. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy is Safe for Patients with Peritoneal Metastases from Gastric Cancer and May Lead to Gastrectomy. Ann Surg Oncol. 2019;26(5):1394-1400.
Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol. 2018;36(19):1922-1929.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697.
Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (AT-TRACTION-4). Ann Oncol. 2019;30(2):250-258.
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471.
Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30(9):1479-1486.
Ding P, Yang P, Tian Y, et al. Neoadjuvant intraperitoneal and systemic paclitaxel combined with apatinib and S-1 chemotherapy for conversion therapy in gastric cancer patients with positive exfoliative cytology: a prospective study. J Gastrointest Oncol. 2021;12(4):1416-1427.
Lv J, Wu J, Wu H, et al. Study protocol of a phase II clinical trial evaluating the efficacy of neoadjuvant intraperitoneal and systemic albumin-bound paclitaxel combined with camrelizumab and S-1 in the treatment of patients with exfoliative cell-positive gastric cancer. Front Oncol. 2023;13:1201928.
Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681-685.
Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63-68.
Van Stein RM, Aalbers A, Sonke GS, et al. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA Oncol. 2021;7(8):1231-1238.
Honore C, Gelli M, Francoual J, et al. Ninety percent of the adverse outcomes occur in 10% of patients: can we identify the populations at high risk of developing peritoneal metastases after curative surgery for colorectal cancer? Int J Hyperthermia. 2017;33(5):505-510.
Hallam S, Tyler R, Price M, et al. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. 2019;3(5):585-594.
National Health and Family Planning Commission Medical Administration Bureau of the People's Republic of China. China colorectal cancer diagnosis and treatment guidelines (2017 edition). Chinese Journal of Practical Surgery. 2018;38(10):1089-1103.
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737-3743.
Botrel T, Clark L, Paladini L, et al. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2016;16:677.
Zheng Y, Zhang J, Chen C, et al. Prophylactic hyperthermic intraperitoneal chemotherapy in T4 colorectal cancer: Can it improve the oncologic prognosis? -A propensity score matching study. Eur J Surg Oncol. 2024;50(2):107958.
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(28):3460-3473.
Safra T, Grisaru D, Inbar M, et al. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients-a case-control study. J Surg Oncol. 2014;110(6):661-665.
Sioulas V D, Schiavone M B, Kadouri D, et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? [J]. Gynecol Oncol, 2017, 145(1): 15-20.
Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study [J]. Ann Surg Oncol, 2015, 22(5): 1570-1575.
Chinese Anti-Cancer Association Cervical Cancer Professional Committee. Expert Consensus on the Clinical Application of Intraperitoneal Hyperthermic Chemotherapy for Gynecological Tumors (2024 Edition) [J]. Chinese Journal of Practical Gynecology and Obstetrics, 2024, 40(01): 62-67.
Van Driel W J, Koole S N, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer [J]. N Engl J Med, 2018, 378(3): 230-240.
Lei Z, Wang Y, Wang J, et al. Evaluation of Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer [J]. JAMA Netw Open, 2020, 3(8): e2013940.
Aronson SL, Lopez-Yurda M, Koole SN, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): final survival analysis of a randomised, controlled, phase 3 trial [J]. Lancet Oncol. 2023; 24(10): 1109-1118.
Arjona-Sánchez A, Espinosa-Redondo E, Gutiérrez-Calvo A, et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial [J]. JAMA Surg. 2023; 158(7): 683-691.
Lim MC, Chang SJ, Park B, et al. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial [J]. JAMA Surg. 2022; 157(5): 374-383.
Falandry C, Rousseau F, Mouret-Reynier M A, et al. Efficacy and Safety of First-line Single-Agent Carboplatin vs Carboplatin Plus Paclitaxel for Vulnerable Older Adult Women With Ovarian Cancer: A GINECO/GCIG Randomized Clinical Trial [J]. JAMA Oncol, 2021, 7(6): 853-861.
Pignata S, Scambia G, Ferrandina G, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial [J]. J Clin Oncol, 2011, 29(27): 3628-3635.
Burger R A, Brady M F, Bookman M A, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer [J]. N Engl J Med, 2011, 365(26): 2473-2483.
Burger R A, Brady M F, Rhee J, et al. Independent radiologic review of the Gynecologic Oncology Group Study 0218, a phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer [J]. Gynecol Oncol, 2013, 131(1): 21-26.
Chang J S, Kim S W, Kim Y J, et al. Involved-field radiation therapy for recurrent ovarian cancer: Results of a multi-institutional prospective phase II trial [J]. Gynecol Oncol, 2018, 151(1): 39-45.
Chan CY, Li H, Wu MF, et al. A Dose-Finding Trial for Hyperthermic Intraperitoneal Cisplatin in Gynecological Cancer Patients Receiving Hyperthermic Intraperitoneal Chemotherapy [J]. Front Oncol. 2021; 11:616264.
Wu MF, Cheng XY, Wang DY, et al. Determining the maximum tolerated dose of paclitaxel combined with a fixed dose of cisplatin for hyperthermic intraperitoneal chemotherapy in ovarian cancer: A multicenter phase I trial [J]. Gynecol Oncol. 2024; 181: 125-132.
You ZY, Wu MF, Li H, et al. A phase I dose-finding trial of hyperthermic intraperitoneal docetaxel combined with cisplatin in patients with advanced-stage ovarian cancer [J]. J Gynecol Oncol. 2024; 35(1): e1.
Gouy S, Ferron G, Glehen O, et al. Results of a multicenter phase I dose-finding trial of hyperthermic intraperitoneal cisplatin after neoadjuvant chemotherapy and complete cytoreductive surgery and followed by maintenance bevacizumab in initially unresectable ovarian cancer [J]. Gynecol Oncol. 2016; 142(2): 237-242.
Brown AP, Jhingran A, Klopp AH, et al. Involved-field radiation therapy for locoregionally recurrent ovarian cancer [J]. Gynecol Oncol. 2013; 130(2): 300-305.
Moran B, Baratti D, Yan T D, et al. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei) [J]. J Surg Oncol, 2008, 98(4): 277-282.
Kusamura S, Barretta F, Yonemura Y, et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery [J]. JAMA Surg, 2021, 156(3): e206363.
Govaerts K, Lurvink RJ, De Hingh IHJT, et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment [J]. Eur J Surg Oncol. 2021; 47(1): 11-35.
Yang R, Fu YB, Li XB, et al. Long-term survival in patients with PMP: a single-institutional retrospective study from China [J]. World J Surg Oncol. 2023; 21(1): 347.
Zhang Y, Zhao X, Gao C, et al. Treatment outcome analysis of bevacizumab combined with cyclophosphamide and oxaliplatin in advanced pseudomyxoma peritonei [J]. World J Gastrointest Surg. 2023; 15(6): 1149-1158.
Lei Ziying, Ding Binghui, Wu Qiyue, et al. Efficacy analysis of cytoreductive surgery combined with intraperitoneal hyperthermic chemotherapy in the treatment of pseudomyxoma peritonei [J]. Chinese Journal of Gastrointestinal Surgery, 2023, 26(12): 1179-1186.
Amblard I, Mercier F, Bartlett DL, et al. Cytoreductive surgery and HIPEC improve survival compared to palliative chemotherapy for biliary carcinoma with peritoneal metastasis: A multi-institutional cohort from PSOGI and BIG RENAPE groups [J]. Eur J Surg Oncol. 2018; 44(9): 1378-1383.
Mehta S, Schwarz L, Spiliotis J, et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study [J]. Eur J Surg Oncol. 2018; 44(11): 1786-1792.
Yu Liu, Qiuyi Huang, Ruijie Wang et al. Efficacy of Hyperthermic Intraperitoneal Chemotherapy Alone for Diffuse Peritoneal Carcinomatosis from Pancreatic Adenocarcinoma: A Single-Centre Retrospective Cohort Study [J]. Research Square. 08 March 2024, PREPRINT (Version 1).
Chinese Anti-Cancer Association. Expert consensus on standardized diagnosis and treatment of hilar cholangiocarcinoma (2015) [J]. Chinese Journal of Hepatobiliary Surgery, 2015, 21(8): 505-511.
Pancreatic Cancer Professional Committee of the Chinese Anti-Cancer Association. Comprehensive Diagnosis and Treatment Guidelines for Pancreatic Cancer (2018 Edition) [J]. Chinese Journal of Surgery, 2018, 56(7): 481-494.
Expert Committee for the Compilation of the Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition), Zhou Jian. Diagnosis and Treatment Guidelines for Primary Liver Cancer (2024 Edition) [J]. Chinese Clinical Medicine, 2024, 31(2): 277-334.
Medical Administration Bureau of the National Health Commission of the People's Republic of China. Diagnosis and Treatment Guidelines for Pancreatic Cancer (2022 Edition) [J]. Chinese Journal of Digestive Surgery, 2022, 21(9): 1117-1136.
Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001) [J]. Signal Transduct Target Ther. 2023; 8(1): 58.
Feng F, Gao Q, Wu Y, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy vs. cytoreductive surgery alone for intrahepatic cholangiocarcinoma with peritoneal metastases: A retrospective cohort study [J]. Eur J Surg Oncol. 2021; 47(9): 2363-2368.
Qin X, Yang T, Xu H, et al. Dying tumor cells-inspired vaccine for boosting humoral and cellular immunity against cancer [J]. J Control Release. 2023; 359: 359-372.
Chen C, Jung A, Yang A, et al. Chimeric Antigen Receptor-T Cell and Oncolytic Viral Therapies for Gastric Cancer and Peritoneal Carcinomatosis of Gastric Origin: Path to Improving Combination Strategies [J]. Cancers (Basel). 2023; 15(23): 5661.
Hassan R, Butler M, O'Cearbhaill RE, et al. Mesothelin-targeting T cell receptor fusion construct cell therapy in refractory solid tumors: phase 1/2 trial interim results [J]. Nat Med. 2023; 29(8): 2099-2109.
Kamrani A, Nasiri H, Hassanzadeh A, et al. New immunotherapy approaches for colorectal cancer: focusing on CAR-T cell, BiTE, and oncolytic viruses [J]. Cell Commun Signal. 2024; 22(1): 56.
Jiang G, Ng YY, Tay JCK, et al. Dual CAR-T cells to treat cancers co-expressing NKG2D and PD1 ligands in xenograft models of peritoneal metastasis [J]. Cancer Immunol Immunother. 2023; 72(1): 223-234.
Sangro B, Chan SL, Kelley RK, et al. Four-year overall survival update from the phase III HIMALA⁃YA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma [J]. Ann Oncol. 2024; 35(5): 448-457.
Marmarelis ME, Wang X, Roshkovan L, et al. Clinical Outcomes Associated With Pembrolizumab Monotherapy Among Adults With Diffuse Malignant Peritoneal Mesothelioma [J]. JAMA Netw Open. 2023; 6(3): e232526.
Lu Huaiwu, Xu Dongdong, Zhao Xib, et al. Interpretation of "2024 NCCN Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Clinical Practice Guidelines (First Edition)" [J]. Chinese Journal of Practical Gynecology and Obstetrics, 2024, 40(02): 187-197.
Dilawari A, Shah M, Ison G, et al. FDA Approval Summary: Mirvetuximab Soravtansine-Gynx for FRα-Positive, Platinum-Resistant Ovarian Cancer [J]. Clin Cancer Res. 2023; 29(19): 3835-3840.
BellaÁ, Arrizabalaga L, Di Trani CA, et al. Intraperitoneal administration of a modified vaccinia virus Ankara confers single-chain interleukin-12 expression to the omentum and achieves immune-mediated efficacy against peritoneal carcinomatosis [J]. J Immunother Cancer. 2023; 11(11): e006702.
Di Trani CA, Cirella A, Arrizabalaga L, et al. Intracavitary adoptive transfer of IL-12 mRNA-engineered tumor-specific CD8+T cells eradicates peritoneal metastases in mouse models [J]. Oncoimmunology. 2022; 12(1): 2147317.
Qian S, Chen J, Zhao Y, et al. Intraperitoneal administration of carcinoembryonic antigen-directed chimeric antigen receptor T cells is a robust delivery route for effective treatment of peritoneal carcinomatosis from colorectal cancer in pre-clinical study [J]. Cytotherapy. 2024; 26(2): 113-125.
Ouyang Xiaoch. Application of Traditional Chinese Medicine Characteristic Nursing in Pain Management of Advanced Cancer Patients [J]. Evidence-Based Nursing, 2021, 7(03): 415-418.
Yu Lu. Effects of acupoint massage and emotional nursing intervention on sleep disorders and quality of life in cancer patients [J]. Guangming TCM, 2022, 37(13): 2435-2437.
Clinical practice of traditional Chinese medicine ear acupuncture patch therapy assisting drug analgesia in pain management of advanced cancer patients [C]//Shanghai Society of Nursing. Proceedings of the 5th Shanghai International Nursing Conference (Part 1), 2022: 2.
Lu Y,Xiao Z,Zhao X,et al. Incidence,risk factors,and outcomes of the transition of HIPEC-induced acute kidney injury to acute kidney disease:a retrospective study[J].Ren Fail.2024;46(1):2338482.
Rau B,Lang H,Koenigsrainer A,et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases:The Phase III GAS-TRIPEC-I Trial[J].J Clin Oncol.2024;42(2):146-156.
Kitaguchi D,Park EJ,Baik SH,et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus R0 resection for resectable colorectal cancer with peritoneal metastases and low peritoneal cancer index scores:a collaborative observational study from Korea and Japan[J].Int J Surg.2024;110(1):45-52.
Application value of comprehensive nutritional intervention in conversion therapy of gastric cancer patients with positive peritoneal exfoliated cytology [J]. Parenteral and Enteral Nutrition, 2021, 28(06): 332-337.
Gong Q,Song C,Wang X,et al. Hyperthermic intraperitoneal chemotherapy with recombinant mutant human TNF-α and raltitrexed in mice with colorectal-peritoneal carcinomatosis[J].Exp Biol Med (Maywood).2020,245(6):542-551.
Analysis of the short-term safety and feasibility of intraoperative peritoneal perfusion with recombinant restructured human tumor necrosis factor in patients with advanced colorectal cancer [J]. Chinese Journal of Colorectal Diseases (Electronic Edition), 2024, 13(02): 94-100.