[Medical News] New Treatment Model in Oncology - Intracavitary Hyperthermic Chemotherapy Officially Launched
Recently, our hospital's oncology department successfully introduced a new green model for the auxiliary treatment of malignant tumors in the abdominal cavity—hyperthermic intraperitoneal chemotherapy (HIPEC), and conducted the first case of hyperthermic intraperitoneal chemotherapy for a patient with advanced gastric cancer.
2025-01-06
Recently,Our hospital's oncology department has successfully introduced a new green model for the auxiliary treatment of malignant tumors in the abdominal cavity—Hyperthermic Intraperitoneal Chemotherapy, and has conducted the first case of hyperthermic intraperitoneal chemotherapy for a patient with advanced gastric cancer.The implementation of this new clinical application undoubtedly brings new light and hope to many cancer patients, and also marks a new breakthrough in the hospital's comprehensive prevention and treatment system for malignant tumors in the abdominal cavity, specifically in the key area of auxiliary treatment methods. It meets the clinical treatment needs of "preventing tumor implantation and controlling malignant ascites" and lays a solid foundation for the subsequent construction of a more complete and efficient tumor treatment ecosystem.
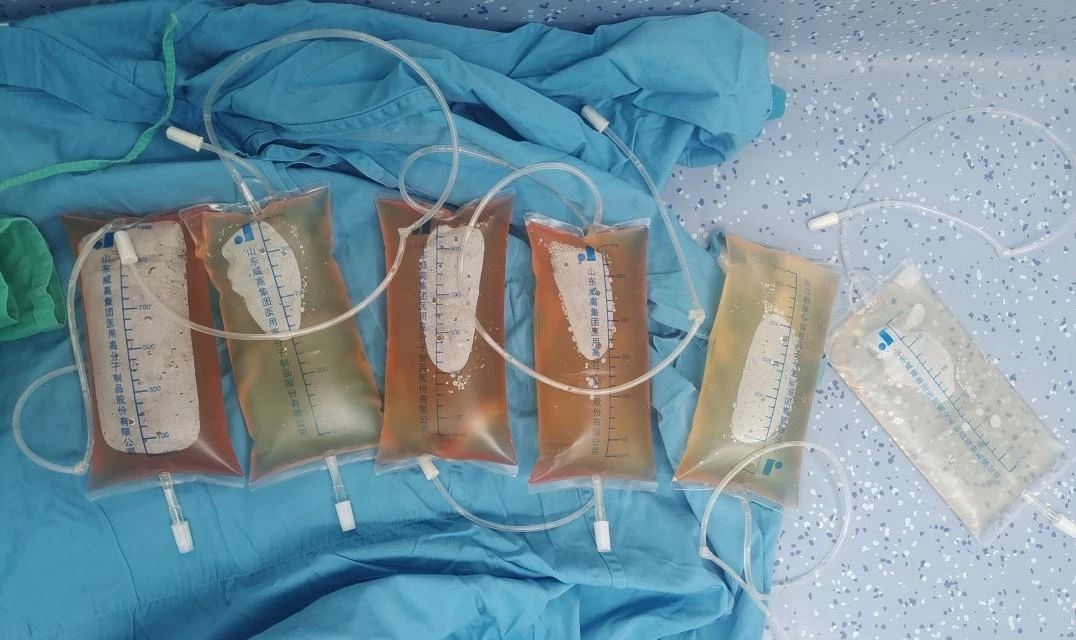
The first case of hyperthermic intraperitoneal treatment in the oncology department.
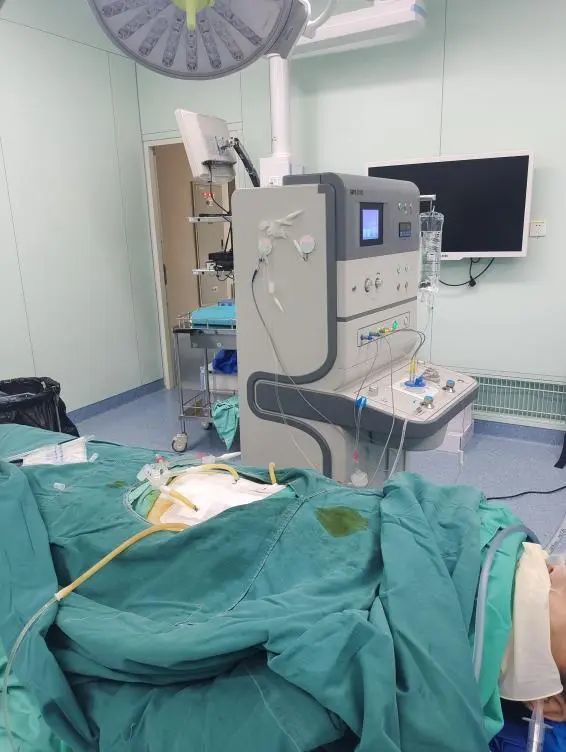
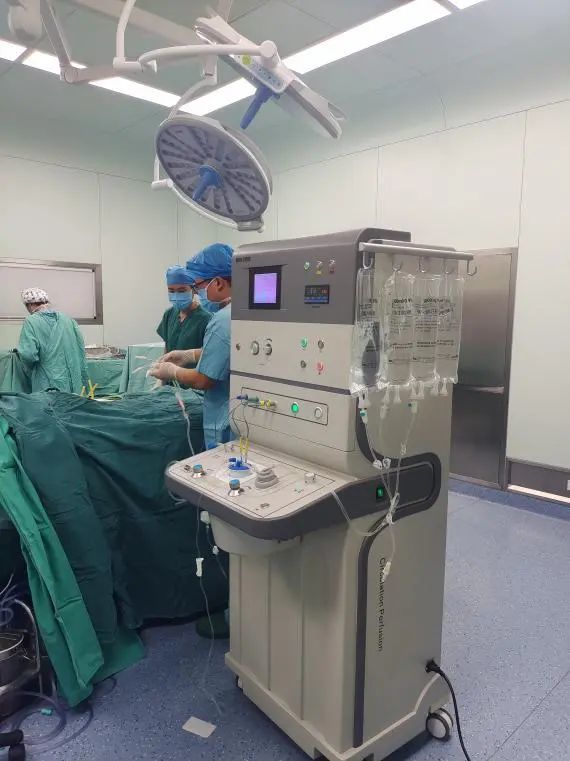
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)As a green innovative therapy that has emerged in recent years in the field of auxiliary treatment for malignant tumors in the abdominal cavity, the clinical treatment effect is significant. This technology uses catheters placed directly during surgery or drainage catheters left in the abdominal cavity postoperatively, or catheters placed through external puncture techniques, to inject heated perfusion fluid into the abdominal cavity. Under the dynamic monitoring and feedback adjustment of a computer, the perfusion fluid maintains a treatment temperature of 42.5°C for 60 minutes, and sensitive chemotherapy drugs can be added to achieve a synergistic effect between chemotherapy and hyperthermia, effectively killing free cancer cells or small implanted metastatic foci. When used in conjunction with surgery, it can treat or prevent tumor implantation and metastasis, and even when used alone, it can effectively controlmalignant ascites.This improves the quality of life and survival time of patients.
1. Clinical Treatment Advantages
The significant advantages of this technology are reflected in multiple dimensions, such as being green and safe, maintaining constant temperature, chemotherapy synergy, physical flushing, multiple perfusion modes, multi-cavity, micro-injury, preventing metastasis, and treating ascites.
(1) Thermal Effect: Scalding Cancer Cells to Death
Cancer cells are essentially mutated human cells, which are sensitive to heat just like normal human cells, and they are even more heat intolerant than normal cells. Clinical studies have shown that when the temperature is maintained at 42.5°C for 60 minutes, most tumor cells can be killed. This is because high temperatures damage the cell membranes of cancer cells, increasing their permeability, leading to cell death. At the same time, high temperatures can also damage proteins within the cells, obstructing the DNA transcription process of cancer cells, causing them to lose their reproductive ability and ultimately die. Additionally, high temperatures can damage lysosomes within the cytoplasm, and the digestive enzymes released after lysosomal rupture can also cause cell death. 43°C is the golden temperature line for human cells, providing an excellent "treatment window" for selectively killing cancer cells using thermal methods.
(2) Perfusion Effect: Washing Away Cancer Cells
At the same time, circulating hyperthermic perfusion acts like a "cleaner"; after establishing a circulating flushing mechanism within the abdominal cavity, free cancer cells in the abdominal cavity are gradually filtered and removed with the flow of the perfusion fluid. With each successful flushing cycle, the number of free cancer cells in the abdominal cavity shows a significant decreasing trend, as if being "washed" clean by an invisible filter. This safe and green physical flushing effect greatly reduces the residual base of cancer cells in the abdominal cavity and the risk of re-implantation and metastasis.
(3) Chemotherapy Effect: Directly Destroying Cancer Cells
The synergistic effect of chemotherapy involves chemotherapy drugs with heat-sensitizing properties, which significantly increase their sensitivity when exposed to heat, resulting in an exponential increase in efficacy for patients. The human body has a peritoneal-blood barrier, making it difficult for chemotherapy drugs injected into the abdominal cavity to return to the peripheral blood, thus resulting in a higher concentration of chemotherapy drugs in the abdominal cavity, which is more effective in killing tumor cells. Moreover, because the concentration of chemotherapy drugs absorbed into the peripheral blood is extremely low, systemic reactions related to chemotherapy are minimal or nonexistent. During an hour of flushing, the chemotherapy drugs in the abdominal cavity can continuously and stably deliver a comprehensive and deep impact on tumor cells.
(4) Thermal Effect + Perfusion Effect + Chemotherapy Effect: Achieving a Synergistic Effect of 1+1+1>3
The constant temperature perfusion fluid at 42.5°C enters the abdominal cavity; "heat" can not only directly kill tumor cells, but it can also enhance the efficiency of chemotherapy drugs, increasing their depth of penetration and permeability into tumor cells. The concentration of chemotherapy drugs in the abdominal cavity is relatively high, the circulation time is longer, and the concentration returning to the peripheral blood is lower, resulting in high efficiency with low side effects.
The first case of hyperthermic intraperitoneal treatment in the oncology department.
2. Clinical Indications
Gastric cancer, colorectal cancer, treatment for preventing implantation and metastasis during and after surgery;
Perfusion treatment for cancer cells, metastatic nodules, and residual lesions that cannot be removed surgically;
Perfusion treatment for malignant ascites (pleural cavity, abdominal cavity);
Perfusion treatment for ovarian cancer metastases;
Treatment for malignant pleural and peritoneal mesothelioma;
3. Contraindications
Patients with severe adhesions in the thoracic and abdominal cavities;
Intestinal obstruction, liver cirrhosis with ascites;
ECOG > 2, extremely poor physical condition, unable to tolerate hyperthermic perfusion treatment;
Patients with bleeding tendency diseases;
Those with uncontrolled hypertension, heart disease, cerebrovascular accidents, coronary heart disease, and arrhythmias;
Patients with advanced malignant ascites with failure of one or more vital organs such as the heart, lungs, liver, and kidneys;
Patients with inguinal hernia,Laparoscopic herniaPatients;
Patients with poor compliance;
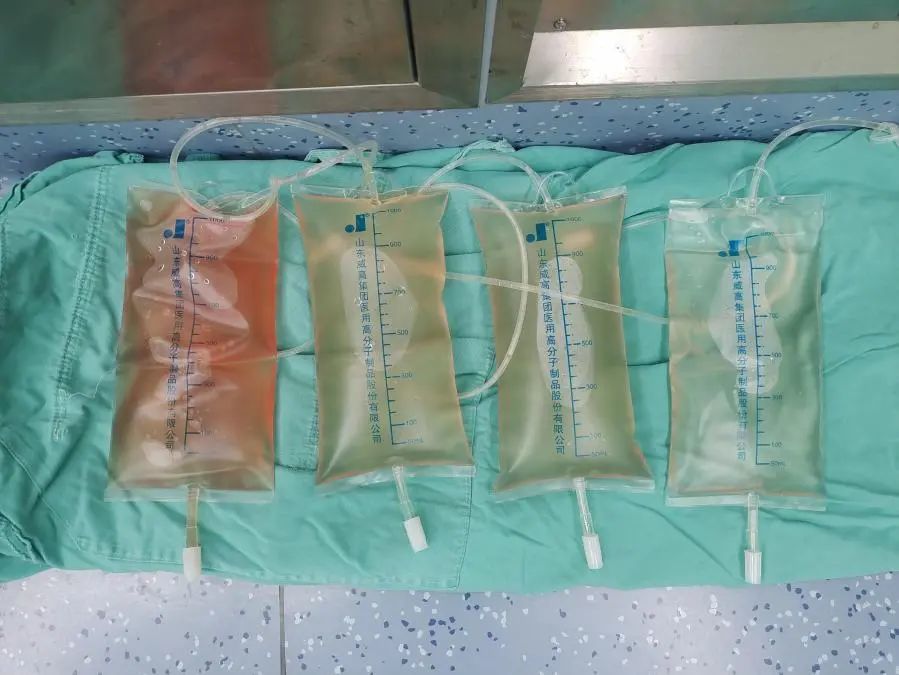
Comparison of effusion before and after the first case of intraperitoneal hyperthermic perfusion therapy in the oncology department
With the official launch of intraperitoneal hyperthermic perfusion chemotherapy technology, the oncology department will continue to be patient-centered, supported by technology, and continuously deepen the research and application of the standard treatment plan for peritoneal cancer combining tumor cell reduction surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) to improve treatment outcomes, prolong life, and enhance quality of life. Hyperthermic intraperitoneal chemotherapy (HIPEC), as a green and safe auxiliary treatment for tumors, has significant advantages in the prevention and treatment of malignant tumor peritoneal implantation metastasis andmalignant ascitestreatment.
Hyperthermic intraperitoneal chemotherapy (HIPEC) is open to the whole hospital. For patients with malignant pleural and abdominal effusion who receive treatment in the oncology department and then return to their original department for other treatments, the oncology department will ensure follow-up support.
Source: Lanzhou First People's Hospital Micro Release
Key words:
Related News